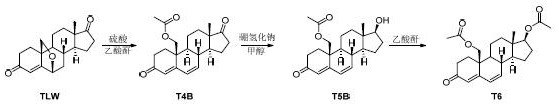
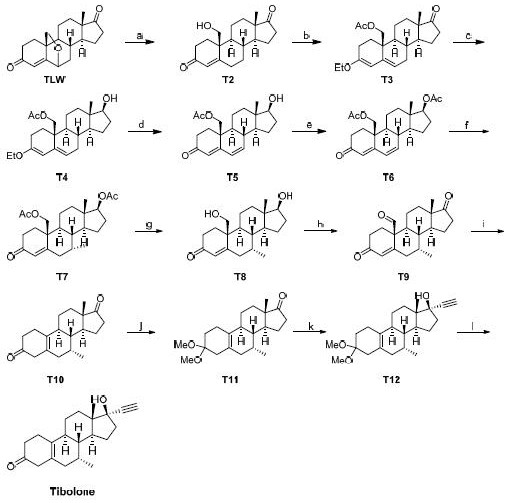
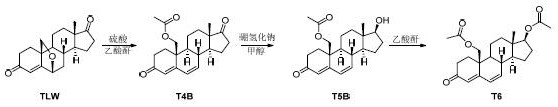
Synthesis method of tibolone intermediate T6
A synthetic method and intermediate technology, applied in the field of preparation of steroidal compounds, can solve the problems of harsh Birch reduction conditions, cumbersome method steps, and high production costs, and achieve environmental protection and large-scale production, avoiding the use of dangerous metal reagents , the effect of shortening the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dissolve TLW (50.0 g, HPLC content ≥ 98%) in acetic anhydride (500 mL) solution, add concentrated sulfuric acid (2.0 g), and stir at room temperature for 6 hours. TLC detects that the reaction is complete, stop the reaction, concentrate to a paste, add ethyl acetate (500 mL) and water (200 mL), separate the layers, extract the aqueous layer with ethyl acetate (200 mL), separate the layers, combine the organic phases, and saturated salt Wash with water (200 mL), separate the layers, dry and concentrate to give T4B as a yellow solid, 57.0 g.
Embodiment 2
[0027] T4B (57.0 g) was dissolved in methanol (570 mL) solution, sodium borohydride (6.3 g) was added, 0 o The reaction was carried out at C for 1 hour, TLC detected that the reaction was complete, adjusted the pH to neutral with dilute hydrochloric acid, concentrated to a paste, added ethyl acetate (570 mL) and water (280 mL), separated, and the aqueous layer was ethyl acetate (280 mL) Extract, separate layers, combine organic phases, wash with saturated brine (280 mL), separate layers, dry, and concentrate to obtain T5B as a yellow solid, 57.0 g.
Embodiment 3
[0029] Dissolve T5B (57.0 g) in dichloromethane (300 mL) solution, add acetic anhydride (57.0 g), add triethylamine (28.5 g), and stir at room temperature for 12 hours. TLC detected that the reaction was complete, adding sodium bicarbonate solution to quench the reaction, filtering, spinning the filtrate to a paste, adding ethyl acetate (570 mL) and water (280 mL), separating the layers, and extracting the aqueous layer with ethyl acetate (280 mL) , separate layers, combine organic phases, wash with saturated brine (280 mL), separate layers, dry, and concentrate to obtain crude yellow solid T6, 62.0 g. The crude product of T6 was placed in methanol (310 mL), heated to reflux, naturally cooled to room temperature, stirred and crystallized, and filtered to obtain T6 as a white solid, 55.8 g, with an HPLC purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com