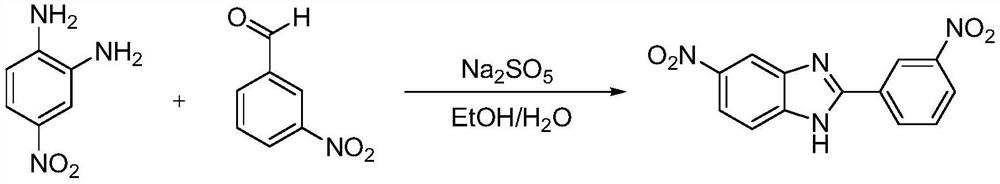
Synthesis process of 5-nitro-2-(3-nitrophenyl) benzimidazole
A synthesis process, a nitrophenyl technology, applied in directions such as organic chemistry, can solve problems such as difficult industrial production, difficult temperature control, serious environmental pollution, etc., and achieve the effects of improving purity, simple and convenient synthesis, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In the present embodiment, the synthetic method of 5-nitro-2-(3-nitrophenyl) benzimidazole comprises the following steps:
[0027] (1) raw material selection, with 4-nitro o-phenylenediamine and 3-nitrobenzaldehyde as reaction raw materials. For selection (EtOH / H 2 O,Na 2 S 2 o 5 ) as a reaction system;
[0028] (2) To synthesize an intermediate, add 10V ethanol (3L) to the reaction flask at room temperature, start stirring, and continue to add 1.1eq of 3-nitrobenzaldehyde (325g) to the system, 10V purified water (3L), 1.1eq Sodium metabisulfite (409.4g), stirred at room temperature for 1h;
[0029] (3) Synthesize the crude product, add 1.0eq of 4-nitro-o-phenylenediamine (300g) to the system, turn on the heating, control the internal temperature at 75°C-80°C, stir the reaction for 46h, monitor the liquid phase (4-nitro-o-phenylenediamine O-phenylenediamine ≤ 3%), 10V purified water (3L) was added dropwise to the system, after the dropwise addition was completed, ...
Embodiment 2
[0035] In the present embodiment, the synthetic method of 5-nitro-2-(3-nitrophenyl) benzimidazole comprises the following steps:
[0036] (1) raw material selection, with 4-nitro o-phenylenediamine and 3-nitrobenzaldehyde as reaction raw materials. For selection (EtOH / H 2 O,Na 2 S 2 o 5 ) as a reaction system;
[0037] (2) To synthesize an intermediate, add 10V ethanol (3L) to the reaction flask at room temperature, start stirring, and continue to add 1.1eq of 3-nitrobenzaldehyde (325g), 10V purified water (3L), 2eq of Sodium metabisulfite (744g), stirred and reacted at room temperature for 1h;
[0038] (3) Synthesize the crude product, add 1.0eq of 4-nitro-o-phenylenediamine (300g) to the system, turn on the heating, control the internal temperature at 75°C-80°C, stir the reaction for 46h, monitor the liquid phase (4-nitro-o-phenylenediamine O-phenylenediamine ≤ 3%), 10V purified water (3L) was added dropwise to the system, after the dropwise addition was completed, the...
Embodiment 3
[0041] In the present embodiment, the synthetic method of 5-nitro-2-(3-nitrophenyl) benzimidazole comprises the following steps:
[0042] (1) raw material selection, with 4-nitro o-phenylenediamine and 3-nitrobenzaldehyde as reaction raw materials. For selection (EtOH / H 2 O,Na 2 S 2 o 5 ) as a reaction system;
[0043] (2) To synthesize an intermediate, add 10V ethanol (3L) to the reaction flask at room temperature, start stirring, and continue to add 1.1eq of 3-nitrobenzaldehyde (325g) to the system, 10V purified water (3L), 1.1eq Sodium metabisulfite (409.4g), stirred at room temperature for 1h;
[0044] (3) Synthesize the crude product, add 1.0eq of 4-nitro-o-phenylenediamine (300g) to the system, turn on the heating, control the internal temperature at 75°C-80°C, stir the reaction for 46h, monitor the liquid phase (4-nitro-o-phenylenediamine O-phenylenediamine ≤ 3%), 10V purified water (3L) was added dropwise to the system, after the dropwise addition was completed, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com