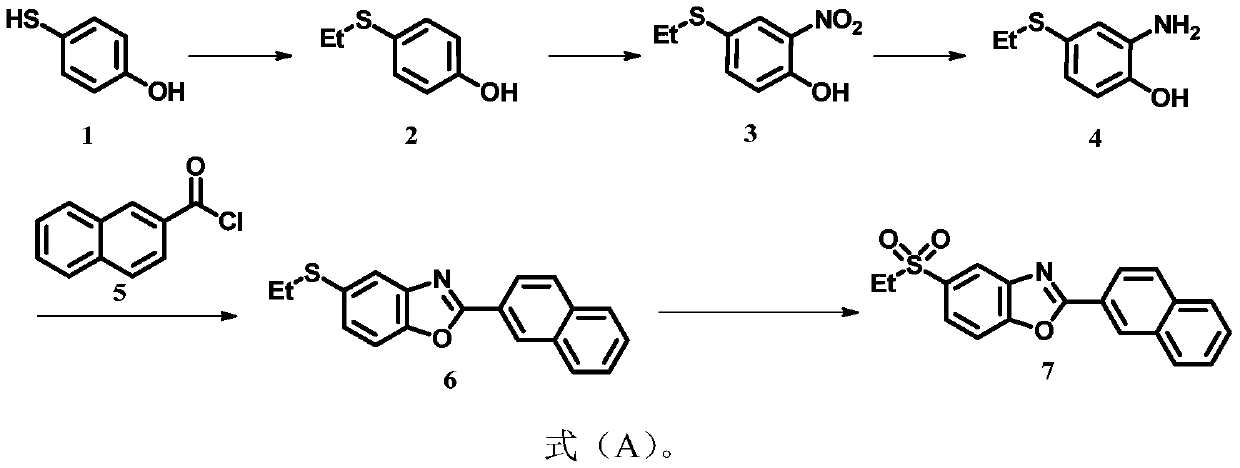
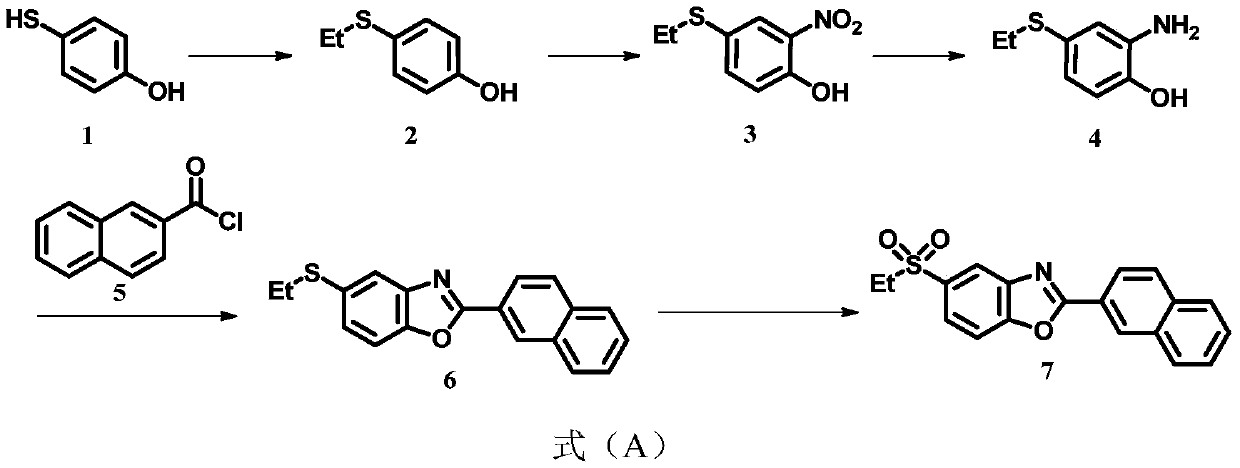
Synthesis process of ezutromid
A synthetic process, the technology of Yezhu Yemi, which is applied in the field of organic chemical synthesis, can solve the problems of high energy consumption and low condensation yield, and achieve the effects of high yield, easy quality and quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Put 12.6g (100mmol) of compound 1,11.7g (110mmol) of sodium carbonate, 100mL of dichloromethane into the substitution kettle, slowly add 15.6g (130mmol) of ethyl iodide at 0°C, transfer to room temperature and stir for 10h. Filtration, solvent distillation, and column chromatography to obtain 11.55 g of compound 2 (yield 75%);
[0036] Take 3.084g (20mmol) of compound 2 in the nitrification kettle, add 2g of silica gel with a particle size of 300-400 mesh, 2g of distilled water and 60mL of DMSO into the nitrification kettle and place it in an ice water bathtub to cool, then slowly add 2.14g (20mmol) Sulfuric acid and 3.204g (40mmol) potassium nitrite were transferred to room temperature and stirred for 10h, filtered, extracted, dried, and separated by column chromatography to obtain 3.669g of compound 3 (yield 92%);
[0037] Into the reduction kettle, add 2.16g (36.9mmol) of nickel powder and 50mL of dichloromethane, then slowly add dropwise 0.15M sulfuric acid solution, ref...
Embodiment 2
[0043] Put 12.6g (100mmol) of compound 1,15.3g (110mmol) of potassium carbonate, and 100mL of acetone into the substitution kettle, slowly add 14.2g (130mmol) of bromoethane at 0°C, transfer to room temperature and stir for 10h, filter, Solvent distillation and column chromatography to obtain 10.01 g of compound 2 (yield 65%);
[0044] Take 3.084g (20mmol) of compound 2 in the nitrification kettle, add 2g of silica gel with a particle size of 300-400 mesh, 2g of distilled water and 60mL of DMF into the nitrification kettle and place it in an ice water bathtub to cool, then slowly add 0.73g (20mmol) HCl in sequence And 2.760g (40mmol) of sodium nitrite, and transferred to room temperature and stirred for 10h, filtered, extracted, dried, and separated by column chromatography to obtain 3.35g of compound 3 (yield 84%);
[0045] To the reduction kettle, add 2.07g (36.9mmol) manganese powder and 50mL dichloromethane, then slowly add 0.15M sulfuric acid solution dropwise, reflux overnigh...
Embodiment 3
[0049] Put 12.6g (100mmol) of compound 1,15.3g (110mmol) of potassium carbonate, 100mL of acetone into the substitution kettle, slowly add 15.6g (130mmol) of ethyl iodide at 0°C, transfer to room temperature and stir for 10h, filter, Solvent distillation and column chromatography to obtain 10.780 g of compound 2 (yield 70%);
[0050] Take 3.084g (20mmol) of compound 2 in the nitrification kettle, add 2g of silica gel with a particle size of 300-400 mesh, 2g of distilled water and 60mL of acetonitrile into the nitrification kettle and place it in an ice water bathtub to cool, then slowly add 3.842g (20mmol) in sequence Citric acid and 2.760g (40mmol) of sodium nitrite were transferred to room temperature and stirred for 10h, filtered, extracted, dried, and separated by column chromatography to obtain 3.590g of compound 3 (yield 90%);
[0051] Into the reduction kettle, add 2.07g (36.9mmol) iron powder and 50mL ethanol, then slowly add 0.15M hydrochloric acid solution dropwise, reflu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com