Human induced pluripotent stem cell as well as preparation method and application thereof
A technology of pluripotent stem cells and somatic cells, applied in the field of human induced pluripotent stem cells and its preparation, can solve the problem that animal models cannot completely simulate human disease states and therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
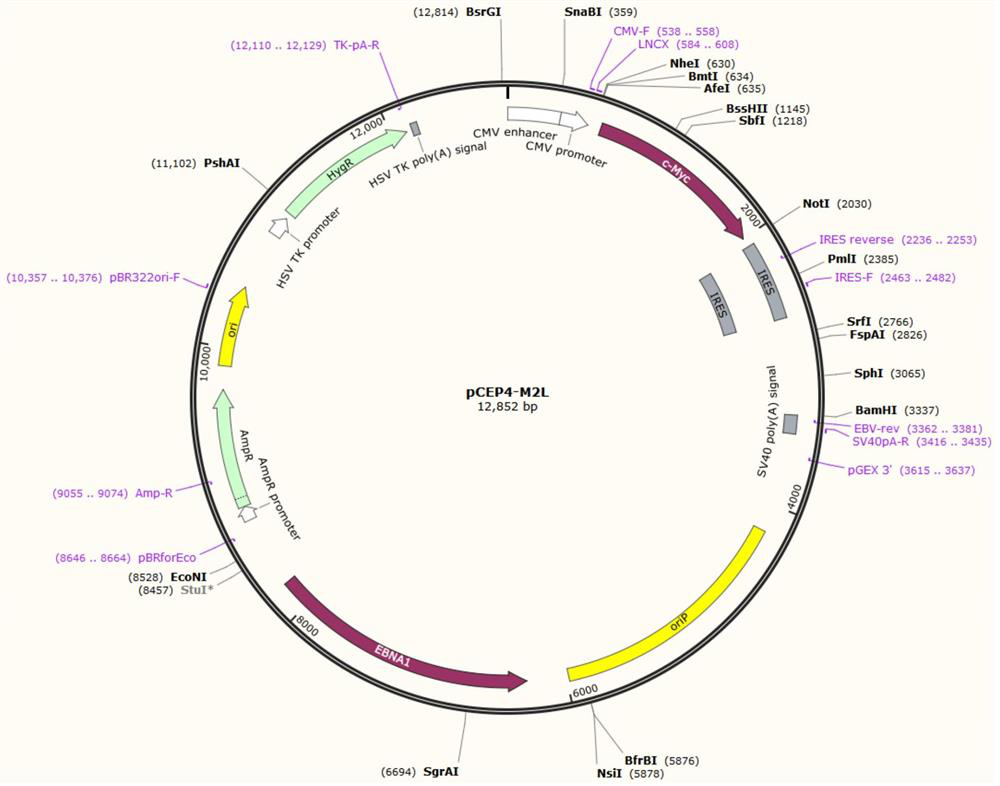
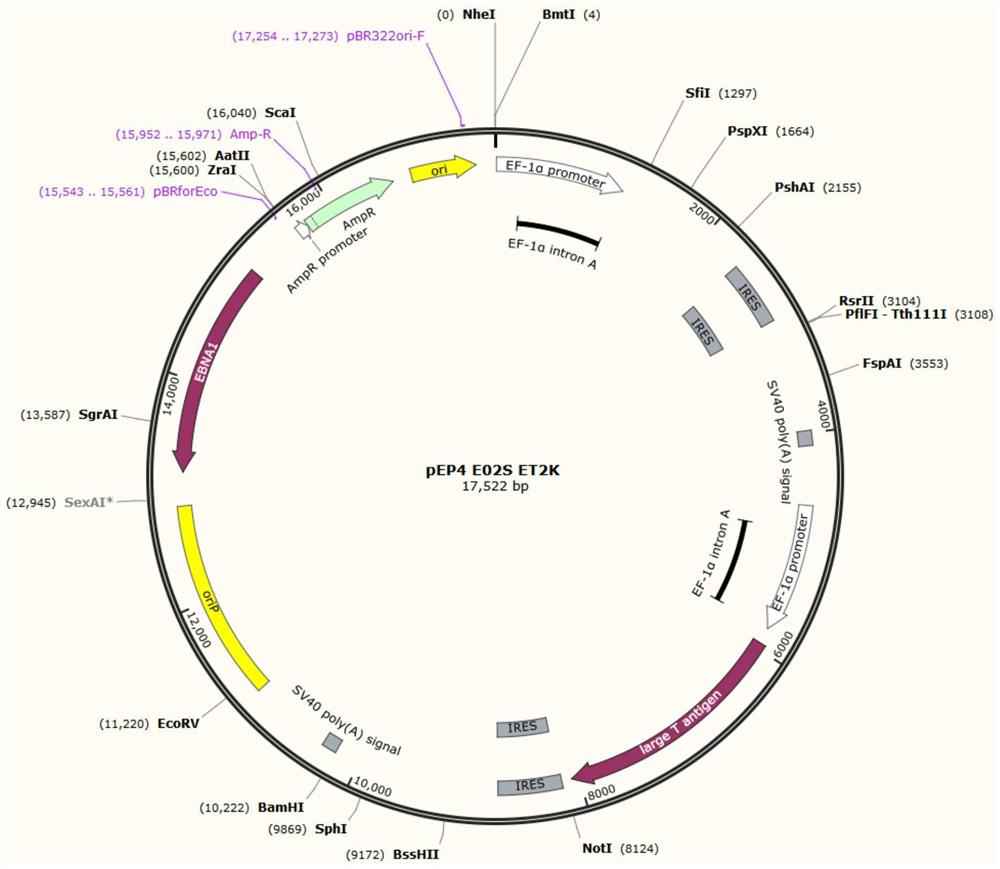
[0029] In some of these embodiments, the preparation method of the human induced pluripotent stem cell line SXMUi001-A-1 includes: the preparation method of the human induced pluripotent stem cell line SXMUi001-A-1 includes:
[0030] Introduce reprogramming factors into somatic cells of hemophilia A patients with c.2440C>T nonsense mutation in the F8 gene; the above-mentioned reprogramming factors include OCT4, SOX2, KLF4, MYC
[0031] Carry out cell reprogramming and obtain by screening.
[0032] In some embodiments, the above-mentioned somatic cells include but are not limited to urine, blood, adipose tissue, amniotic fluid, placental chorion, amniotic membrane, umbilical cord blood, periosteal cells or skin fibroblasts from various parts.
[0033] In some of these embodiments, the above-mentioned somatic cells are urine cells, which avoids the pain of sampling from the skin and the harm to the patient.
[0034] In some of these embodiments, the above-mentioned reprogrammin...
Embodiment 1
[0050] The inventor screened the gene mutation types of hemophilia patients in Shanxi area in the early stage. Among them, a nonsense mutation F8 c.2440C>T was detected in a hemophilia A patient in the Second Hospital of Shanxi Medical University, which was confirmed (http : / / www.factorix.org / ) This mutation is a nonsense mutation.
[0051] 1. Preparation of human induced pluripotent stem cells
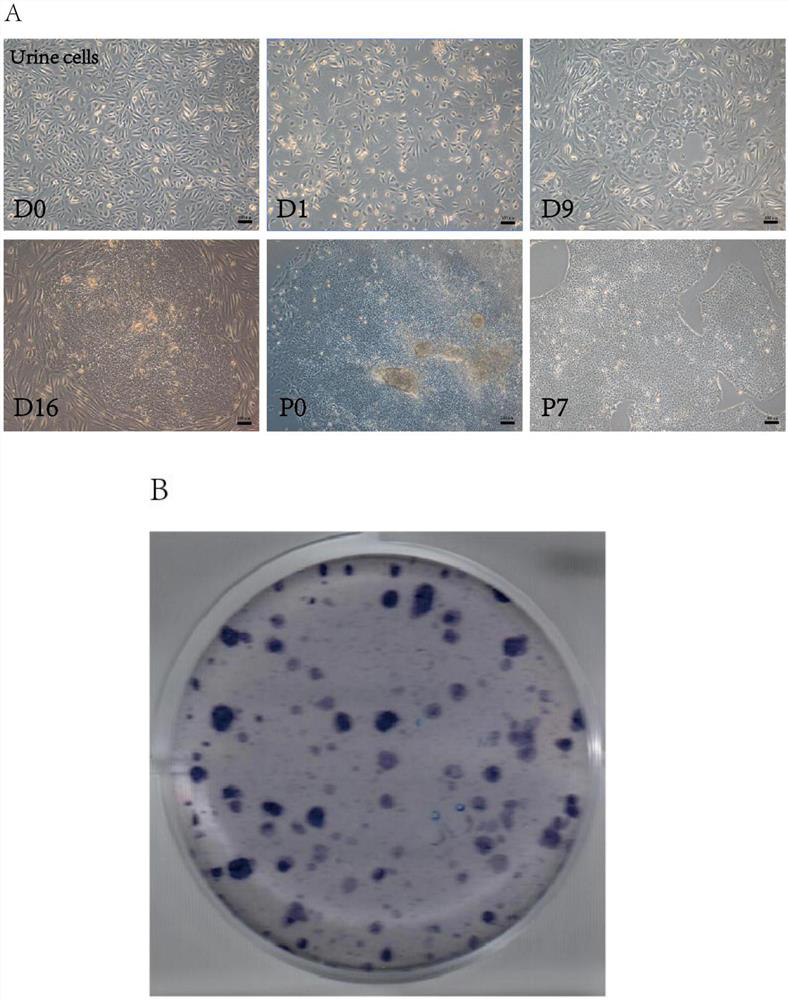
[0052] (1) Collect urine cells:
[0053] With the consent of the patient, urine exfoliated cells were isolated from the urine samples of hemophilia patients, and the urine exfoliated cells were proliferated and cultured [Zhou, T., C. Benda, S. Dunzinger, Y. Huang, J. C. Ho, J. Yang, Y. Wang, Y. Zhang, Q. Zhuang, Y. Li, X. Bao, H.F. Tse, J. Grillari, R. Grillari-Voglauer, D. Pei, and M.A. Esteban. 2012.'Generation of human induced pluripotent stem cells from urine samples', Nat Protoc, 7:2080-9.].
[0054] Collect mid-section urine, centrifuge at 400g for 10 minutes at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com