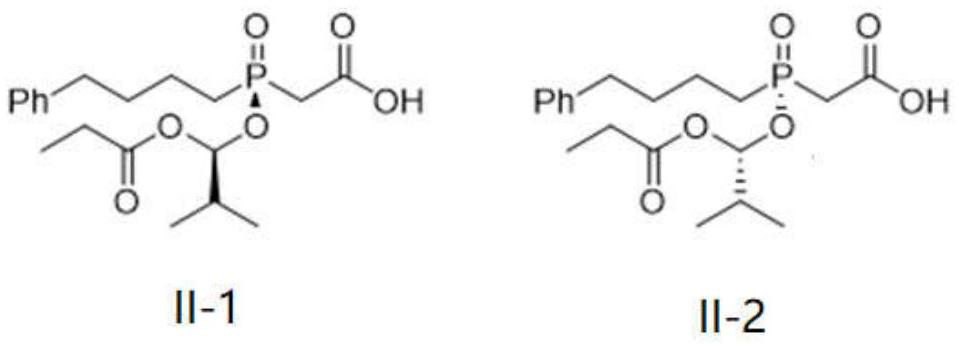
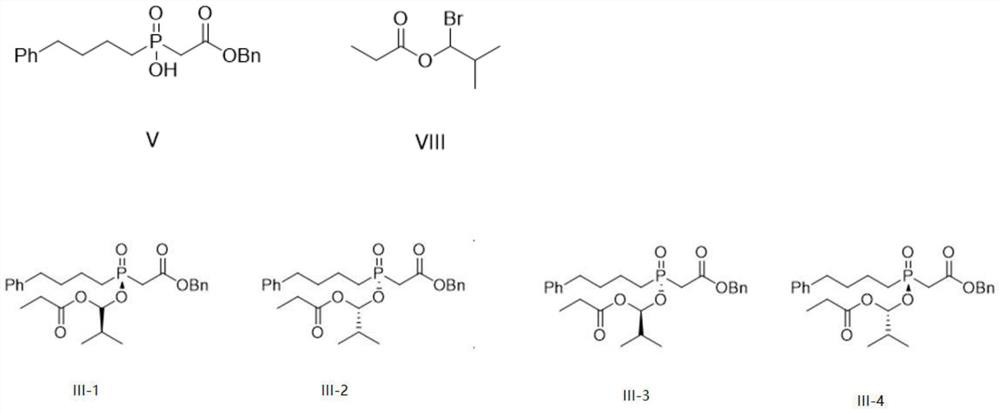
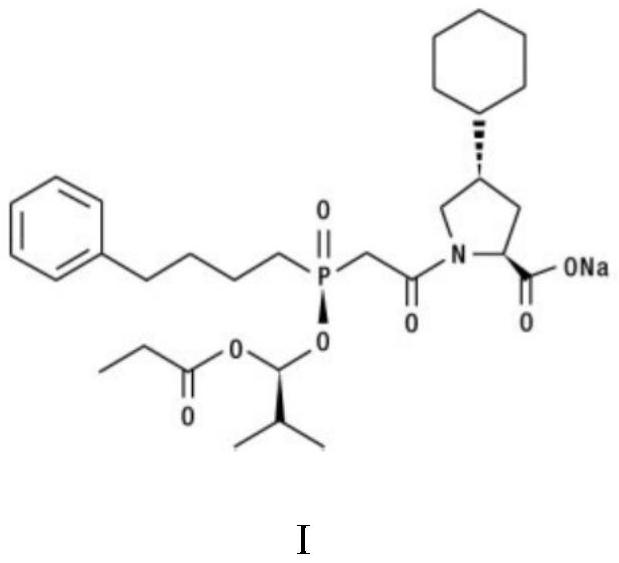
Preparation method of fosinopril sodium intermediate
A technology of fosinopril sodium and intermediates, applied in the field of organic chemical synthesis, can solve the problems of low reaction diastereoselectivity, low yield, low total yield, etc., and achieve the reduction of waste solvent generation, non-reaction High correspondence selectivity, good effect of non-correspondence selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 (screening of reaction base):
[0040] Add 300mL of toluene to a 1L three-necked flask, and then add 54g of the base shown in Table 1, 100g of [hydroxyl (4-phenylbutyl)phosphinyl] benzyl acetate (V) and 1-bromoisobutyl propionate (VIII) 105g was stirred and dissolved, reacted at 25°C for 20-24h, took the reaction solution to detect HPLC, and calculated the dr value of the reaction solution. The results are shown in Table 1:
[0041] Table I
[0042]
[0043]
Embodiment 2
[0044] Embodiment 2 (screening of reaction solvent):
[0045] Add 300mL of the organic solvent shown in Table 2 to a 1L three-necked flask, then throw 54g of N-methylmorpholine, 100g of [hydroxy(4-phenylbutyl)phosphinyl]benzyl acetate (V) and propionic acid 105 g of -1-bromoisobutyl ester (VIII) was stirred and dissolved, and reacted at 25°C for 20-24 hours. The reaction solution was taken for HPLC, and the dr value of the reaction solution was calculated. The results are shown in Table 2:
[0046] Table II
[0047] serial number solvent Reaction liquid dr value 1 Toluene 2.5:1 2 ethyl acetate 2.3:1 3 n-Hexane 2.4:1 4 Dichloromethane 2.2:1
Embodiment 3
[0048] Embodiment 3 (screening of feeding ratio):
[0049] Add 300mL of toluene to a 1L three-necked flask, and then throw N-methylmorpholine, [hydroxy(4-phenylbutyl)phosphinyl]benzyl acetate (V) 100g and propionate-1-bromoisobutyl ( VIII) stirring and dissolving, wherein compound (V): compound (VIII): the mass ratio of N-methylmorpholine is proportioned as shown in Table 3, reacted for 20~24h under the condition of 25 DEG C, and the reaction solution was taken to detect HPLC, The data are shown in Table 3:
[0050] Table three
[0051] serial number Compound (V): compound (VIII): the mass ratio of N-methylmorpholine Reaction liquid dr value 1 1:1.05:0.54 2.5:1 2 1:1.10:0.54 2.3:1 3 1:1.50:0.54 2.3:1 4 1:1.05:0.50 2.2:1 5 1:1.05:1.00 2.2:1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com