Tumor-targeted polypeptide, polypeptide coupling medicine and application of tumor-targeted polypeptide and polypeptide coupling medicine
A tumor-targeting, peptide-coupling technology, applied in the direction of anti-tumor drugs, drug combinations, peptides, etc., can solve the problems of drug dosage limitation, large damage to normal cells, patient drug resistance, and unsatisfactory treatment effects, etc., to achieve Increase the therapeutic effect and avoid the effect of premature degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
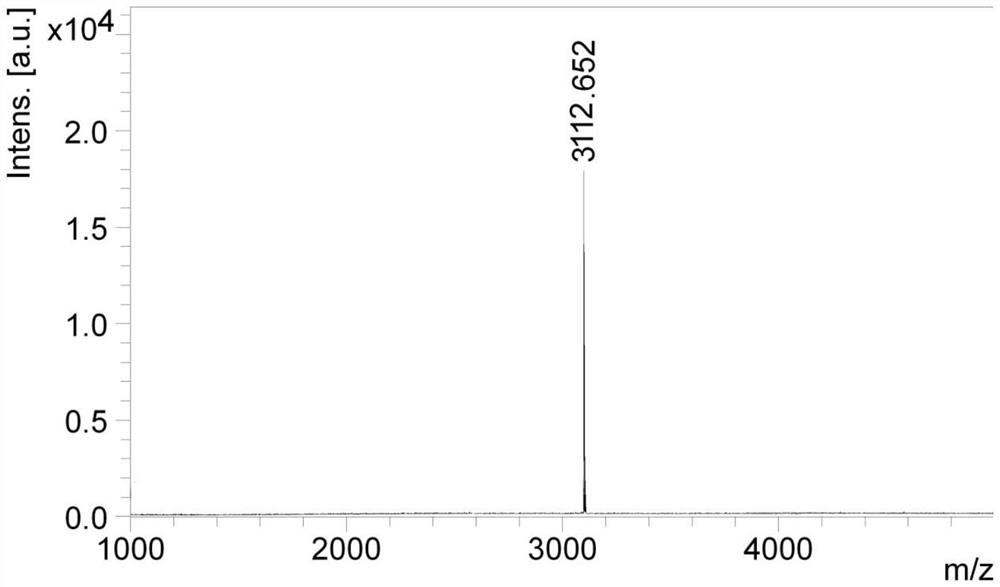
[0045] Example 1: Synthesis of polypeptide sequences (X is none)
[0046] The peptide sequence was synthesized by Fmoc solid-phase peptide synthesis technology. Weigh 20 grams of Wang resin with a substitution degree of 1.0 mmol / g in a solid-phase reaction column, add DMF, and swell with nitrogen gas bubbles for 60 minutes; weigh 6.67 grams (10 mmol) of Fmoc-Asp-ODmab, and 1.6 grams (12 mmol) of HOBt , DMAP 0.12g (1mmol), dissolved in DMF, added 2.3mL DIC (15mmol) in ice-water bath at 0°C, activated for 5 minutes, added to the reaction column, reacted for 1.5 hours, added 14mL acetic anhydride and 12mL pyridine, mixed and blocked for 24 hours , washed three times with DCM, dried the resin after shrinking with methanol, and obtained 26 grams of Fmoc-Asp-ODmab-WangResin in total, and the detection degree of substitution was 0.25 mmol / g.
[0047] Then weigh a total of 16 grams (4 mmol) of the above-mentioned Fmoc-Asp-ODmab-Wang Resin, according to the conventional method of Fmoc...
Embodiment 2
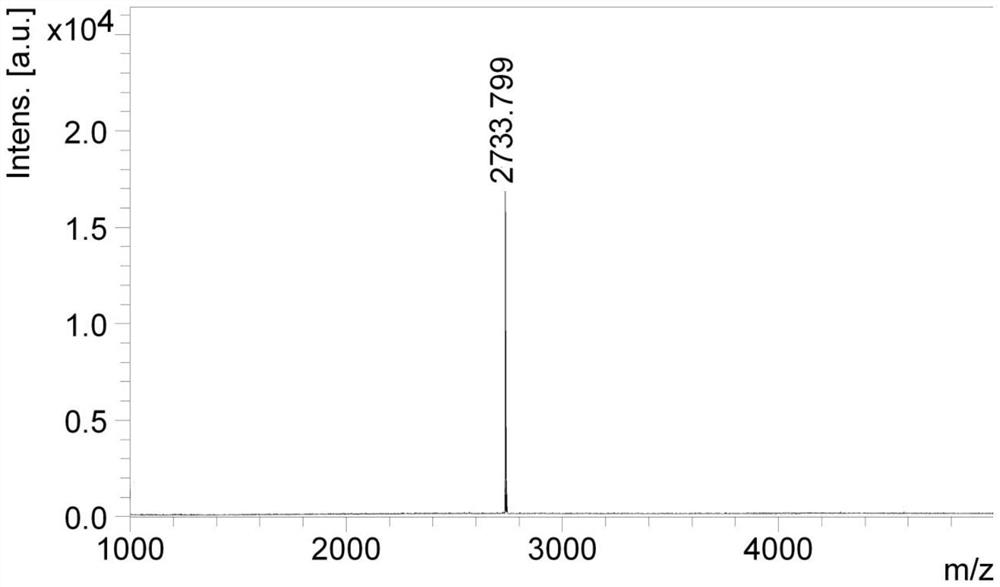
[0051] Embodiment 2: the synthesis of polypeptide sequence (X is Cys)
[0052] Peptide resin was obtained by Example 1, weighed 10.4g (two-thirds, 2mmol) into the solid-phase reaction column, added DMF to swell for 30 minutes, and then continued to couple Boc-Cys according to the standard method of Fmoc solid-phase peptide synthesis (StBu)-OH, after the coupling is completed, add a DMF solution containing 10mmol (5eq) tributylphosphine, react for 1 hour, selectively remove the StBu protecting group of the side chain of the Cys residue, wash with DMF for 5 times, Wash with methanol for 10 minutes and dry in vacuum for 4 hours to obtain Boc-Cys-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf) with exposed Cys side chain mercapto groups. )-Arg(Pbf)-Arg(Pbf)-D-Glu(OtBu)-PEG 4 -Cyclo(Lys-Arg(Pbf)-Gly-Asp-D-Phe)-Wang Resin (where: D-Phe-main chain carboxyl group and Lys main chain amino group form an amide ring; PEG 4 A total of 10.8 g of peptide resins linked to the amino gr...
Embodiment 3
[0053] Embodiment 3: the coupling of paclitaxel (PTX) and polypeptide
[0054] Take by weighing the peptide resin 5.2g (1mmol) that embodiment 1 obtains, add the solid-phase reaction column that has jacket, after DMF swells resin 30 minutes, according to the standard method of Fmoc solid-phase peptide synthesis, continue to couple Fmoc on resin -GABA-OH (Fmoc-γ-aminobutyric acid), remove the Fmoc protecting group, then add DMF solvent, add 5mmol (5eq) of 4-nitrophenyl chloroformate (chloroformic acid-4-nitrophenyl ester), add 2ml pyridine, the reaction temperature was heated to 60°C through a jacket, and the phase reaction was carried out for 6 hours, and the resin was washed 5 times with DMF.
[0055] Add 12mmol of HOBt (12eq), 1mol of DMAP (1eq), 15mmol of DIC (15eq) and 10mmol of paclitaxel (10eq) to the above-mentioned washed peptide resin, add DCM as a reaction solvent, and react at room temperature for 4 hours to obtain paclitaxel Peptide resin coupled to the polypeptid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com