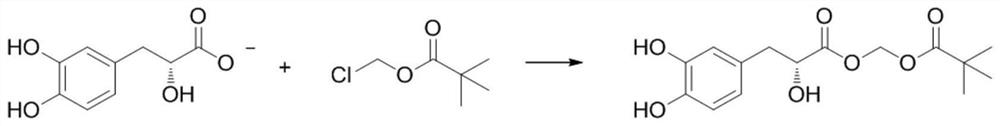
Tanshensu methyl pivalate derivative and preparation method thereof
A technology for methyl pivalate and chloromethyl pivalate, applied in the field of danshensu methyl pivalate derivatives and preparation thereof, can solve problems such as low bioavailability of danshensu, and achieve improved bioavailability , the effect of high reaction efficiency and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 Danshensu methyl pivalate derivatives
[0031] Weigh 110mg of danshensu sodium and dissolve it in 5mL of DMF, stir to dissolve. 214 μL of chloromethyl pivalate (3 eq) and 34 mg of imidazole (1 eq) were added to the reaction. N 2 Under the conditions, react at 60° C. for 12 h, and detect the reaction by TLC. It was extracted three times with saturated brine / ethyl acetate system, and the organic layer was dried over anhydrous sodium sulfate and concentrated. The crude product was purified by silica gel column chromatography to obtain 87 mg of danshensu methyl pivalate derivative with a yield of 56%.
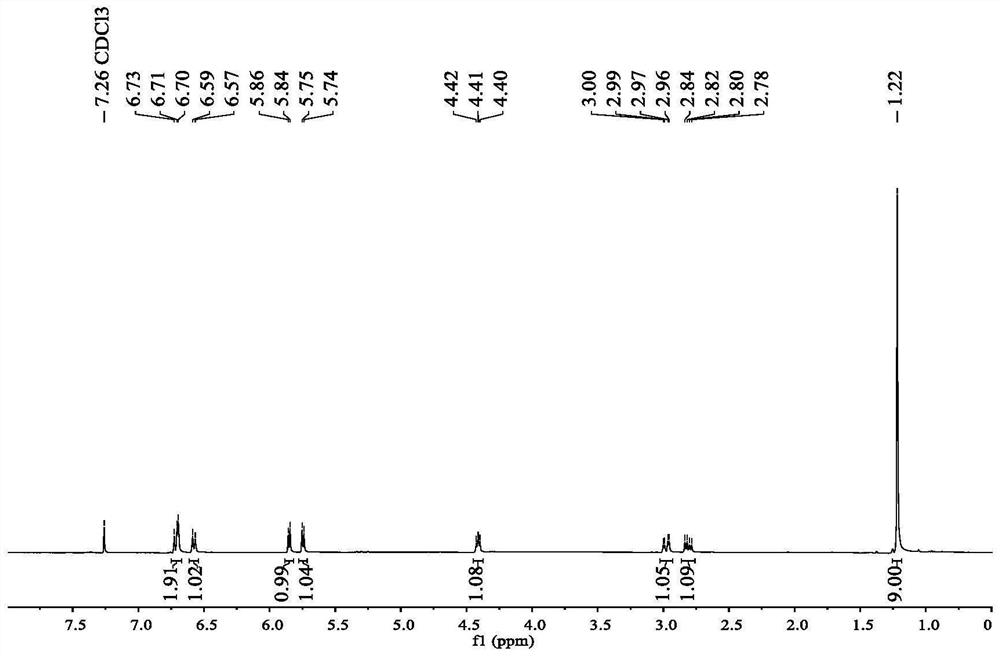
[0032] The NMR data of the prepared Danshensu-methyl pivalate derivatives are as follows:
[0033] 1 H NMR (400MHz, CDCl 3 )δ6.75–6.67(m,2H),6.58(d,J=8.0Hz,1H),5.88–5.82(m,1H),5.75(d,J=5.4Hz,1H),4.45–4.37(m ,1H),2.98(dd,J=14.1,4.2Hz,1H),2.81(dd,J=14.1,6.7Hz,1H),1.22(s,9H).
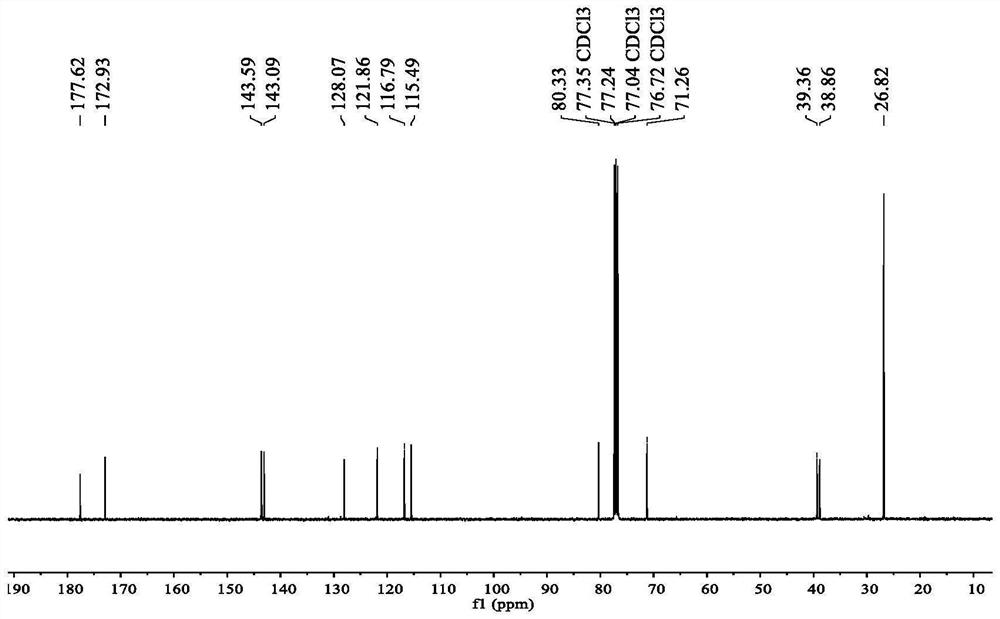
[0034] 13 C NMR (101MHz, CDCl 3 )δ177.62, 172.93, 143.59, 1...
experiment example
[0035] Experimental example Drug bioavailability experiment
[0036] 18 SD rats were randomly divided into the experimental group of danshensu pivalate methyl ester derivatives, the danshensu control group and the danshensu palmitate control group, with 6 rats in each group; the mice in each group were fed with food after 12 hours of fasting before the experiment. Stomach administration. Blood was collected from the canthus at 15, 30 minutes and 1, 2, 4, 6, 8, 12, 16, 20, and 24 hours after intragastric administration, and then put into a heparin-treated centrifuge tube, centrifuged and drawn In the upper layer of plasma, LPLC-MS method was used to measure the content of Danshensu in the plasma, and the blood concentration of Danshensu was calculated, and the blood concentration-time curve of Danshensu was made according to the blood concentration, and then the AUC was calculated by the trapezoidal method; The larger the value, the higher the bioavailability of the drug; the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com