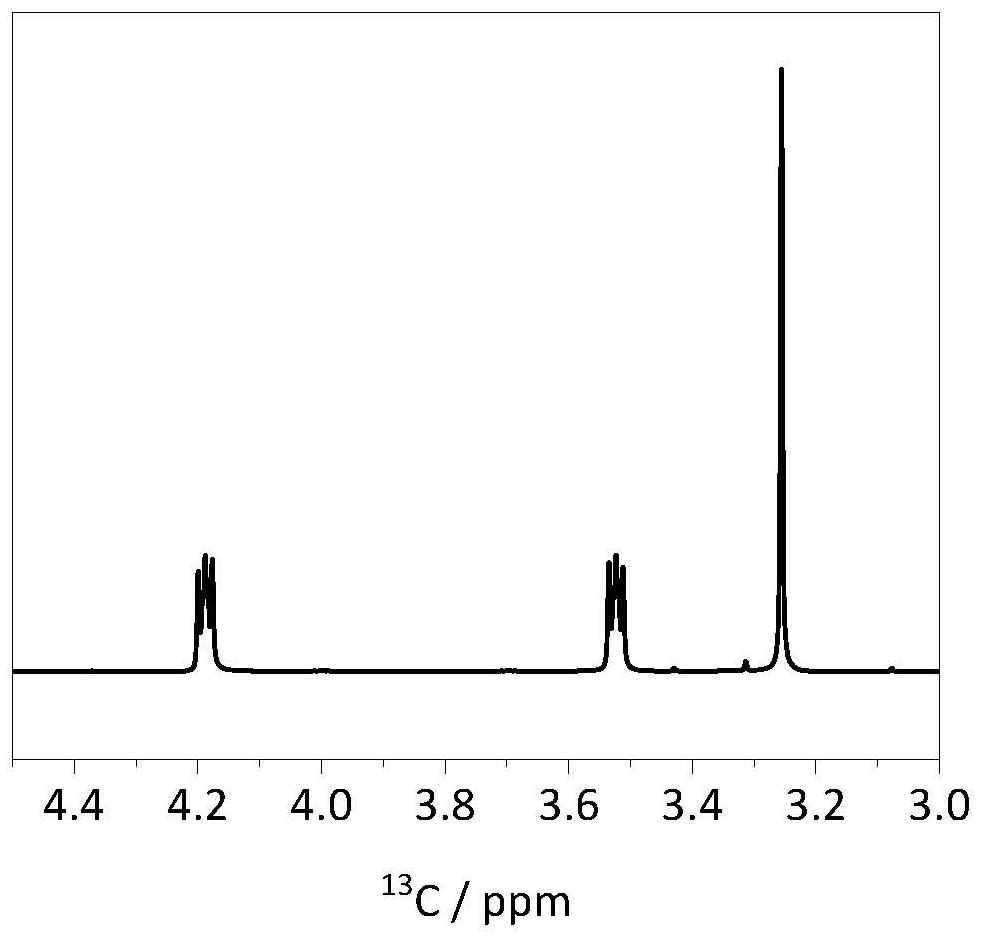
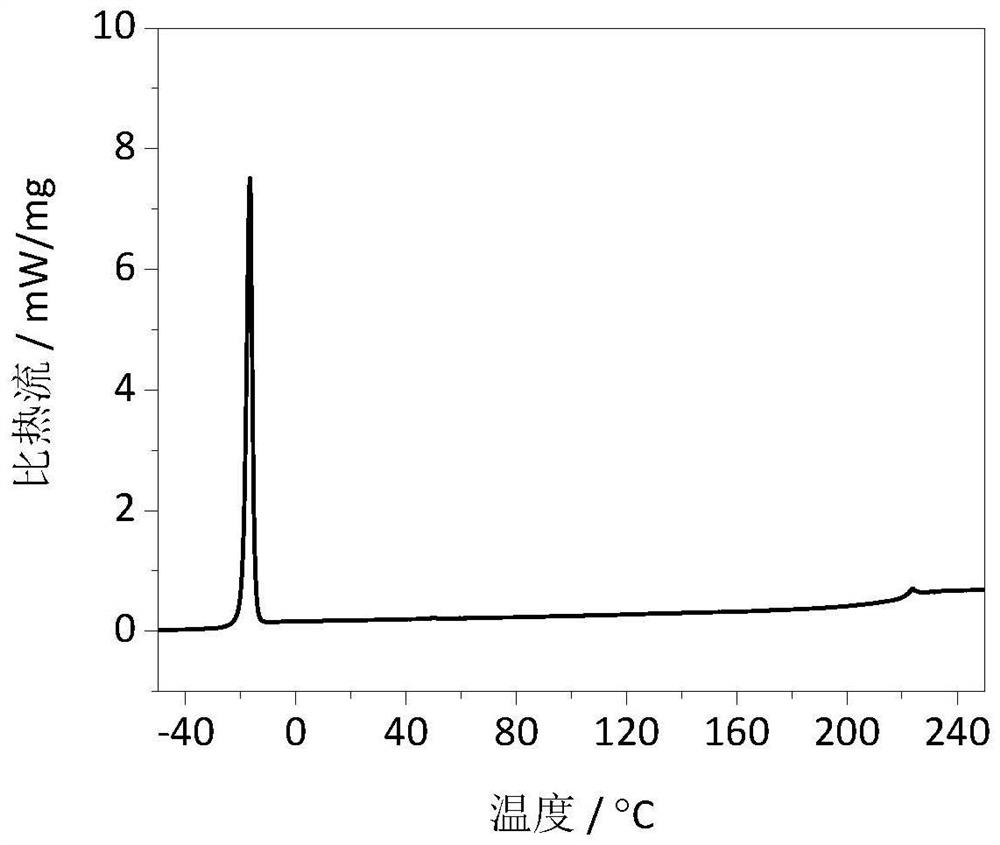
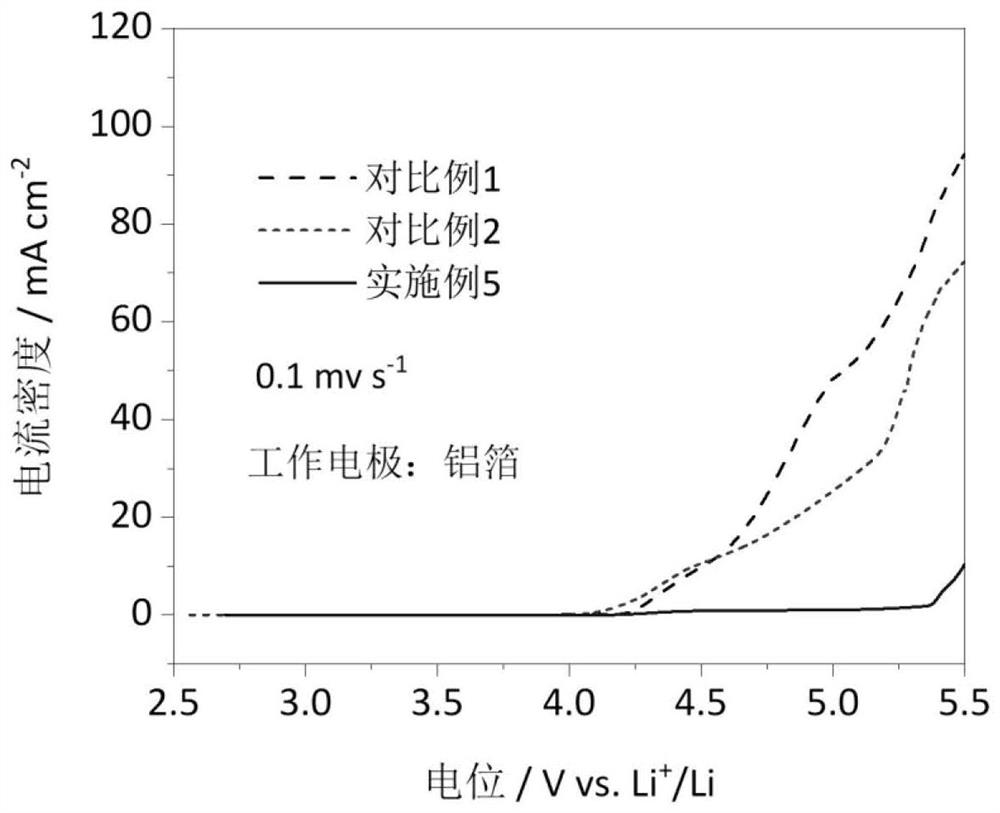
Carbonic ester-based electrolyte with ether oxygen bond functional group and application of carbonic ester-based electrolyte
A carbonate-based, electrolyte technology, applied in the field of lithium-ion batteries, can solve the problems of not meeting the safety requirements of energy storage devices, limiting practical applications, ether solvents with low boiling point and flash point, and achieving shortened self-extinguishing time, Improved Coulombic efficiency, cycle life, and high flash point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) In a glove box filled with argon (H 2 O2 Molecular sieves remove water from solvents. At room temperature at 25°C, dissolve lithium bisfluorosulfonyl imide (LiFSI) as a single lithium salt in the solvent at a concentration of 1 mol / L, and stir evenly to obtain the basic electrolyte containing compound 1 provided by the present invention .
[0037] (2) Add lithium nitrate (LiNO 3 ) additive to obtain the electrolyte solution of Example 1 containing Compound 1 provided by the present invention. Among them, lithium nitrate (LiNO 3 ) is added in an amount of 1% of the total mass of the electrolyte.
Embodiment 2
[0039] (1) In a glove box filled with argon (H 2 O2 Molecular sieves remove water from solvents. At room temperature at 25°C, dissolve lithium bisfluorosulfonyl imide (LiFSI) as a single lithium salt in the solvent at a concentration of 1 mol / L, and stir evenly to obtain the basic electrolyte containing compound 1 provided by the present invention .
[0040] (2) Add lithium difluorobisoxalate phosphate (LiDFBOP) additive to the basic electrolyte prepared in step (1) to obtain the electrolyte of Example 2 containing compound 1 provided by the present invention. Wherein, the added amount of lithium difluorobisoxalate phosphate (LiDFBOP) accounts for 1% of the total mass of the electrolyte.
Embodiment 3
[0042] (1) In a glove box filled with argon (H 2 O2 Molecular sieves remove water from solvents. At room temperature at 25°C, dissolve lithium bisfluorosulfonyl imide (LiFSI) as a single lithium salt in the solvent at a concentration of 1 mol / L, and stir evenly to obtain the basic electrolyte containing compound 1 provided by the present invention .
[0043] (2) Add lithium nitrate (LiNO 3 ) and lithium difluorobisoxalate phosphate (LiDFBOP) additive to obtain the electrolyte solution of Example 3 containing compound 1 provided by the present invention. Among them, lithium nitrate (LiNO 3 ) and lithium difluorobisoxalate phosphate (LiDFBOP) both accounted for 1% of the total mass of the electrolyte.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com