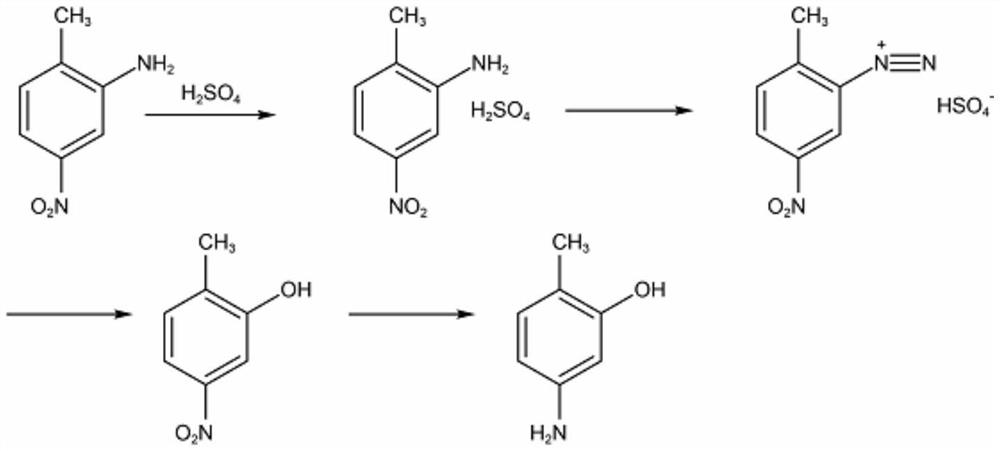
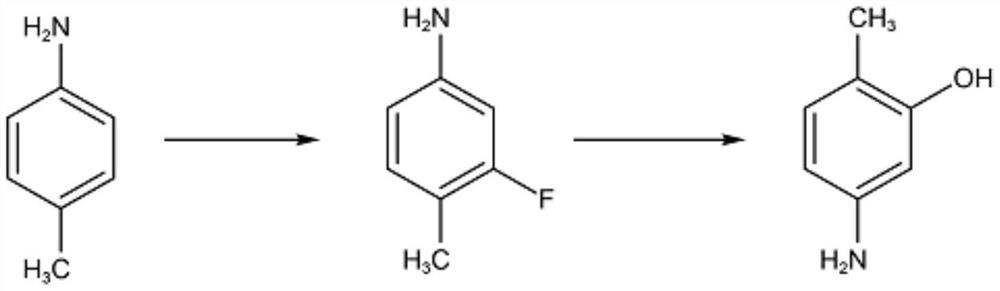
Preparation method of 2-methyl-5-aminophenol
A technology of aminophenol and methyl nitrobenzene is applied in the preparation of amino hydroxy compounds, the preparation of organic compounds, chemical instruments and methods, etc. Simple and safe, good economy, mild reaction process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Nucleophilic substitution reaction. Weigh 22.00g (0.55mol) sodium hydroxide, 59.40g (0.55mol) benzyl alcohol (nucleophilic reagent), 200.00g toluene and add in the four-necked flask to carry out mixed reaction, heat up to reflux and separate out wherein water, and then the temperature of the reaction material was lowered to 10-20°C, and a toluene solution of 3-chloro-4-methylnitrobenzene (85.75g / 0.50mol) was added dropwise, and the reaction temperature was controlled at 10-20°C during the dropping process. During the dropwise addition, red solids were formed in the reaction material. After the dropwise addition, the temperature in the kettle was maintained at 10-20°C for 3.0 hours. The reaction material was sampled for HPLC (high performance liquid chromatography) control analysis. When the raw material in the reaction material was 3 When the content of -chloro-4methylnitrobenzene is less than 1.0%, the reaction is qualified, and then the reaction material is cooled...
Embodiment 2
[0043](1) Nucleophilic substitution reaction. Weigh 32.42g (0.55mol) 95% potassium hydroxide, 59.40g (0.55mol) benzyl alcohol (nucleophilic reagent), 200.00g toluene and add in the four-necked flask, after being warmed up to reflux, separate out wherein water, and then the temperature of the reaction material was lowered to 10-20°C, and a toluene solution of 3-chloro-4-methylnitrobenzene (85.75g / 0.50mol) was added dropwise, and the reaction temperature was controlled at 10-20°C during the dropping process. During the dropwise addition, red solids were formed in the reaction material. After the dropwise addition, the temperature in the kettle was maintained at 10-20°C for 3.0 hours, and samples were taken for HPLC (high performance liquid chromatography) control analysis. When the raw material 3-chloro When the content of -4 methylnitrobenzene < 1.0%, the reaction is qualified, then the reaction material is cooled to 0 ~ 5 ° C for 1.0 h of heat preservation and crystallization,...
Embodiment 3
[0047] (1) Nucleophilic substitution reaction. Weigh 22.00g (0.55mol) sodium hydroxide, 59.40g (0.55mol) benzyl alcohol (nucleophile), 200.00g toluene and add in the four-necked flask, heat up to reflux and separate the water therein through the water separator, Finally, the temperature of the reaction material was lowered to 10-20°C, and a toluene solution of 3-chloro-4-methylnitrobenzene (85.75g / 0.50mol) was added dropwise. During the addition process, a red solid was formed in the reaction material. After the dropwise addition, the temperature in the kettle was maintained at 10-20°C for 3.0 hours, and samples were taken for HPLC (high performance liquid chromatography) control analysis. When the raw material 3-chloro When the content of -4 methylnitrobenzene < 1.0%, the reaction is qualified, then the reaction material is cooled to 0 ~ 5 ° C for 1.0 h of heat preservation and crystallization, filtered after heat preservation and crystallization, and the filter cake is washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com