Targeting chimera for degrading ERK1/2 protein and application thereof
A chimera and targeting technology, applied in drug combinations, peptide preparation methods, peptides, etc., to achieve good application prospects, inhibit tumor growth, and solve clinical problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
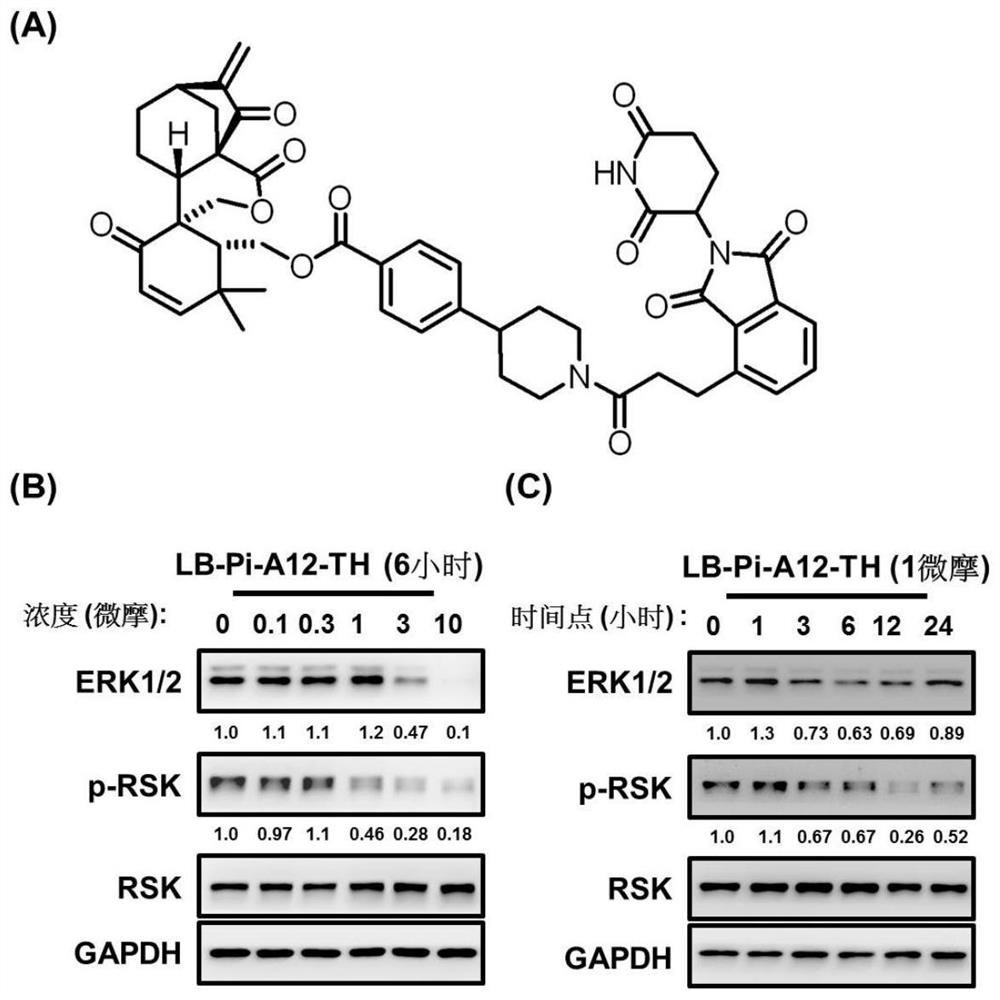
[0095] A targeting chimera (represented by LB-Pi-A12-TH), whose structure is as follows figure 1 As shown in A, its ERK1 / 2 degradation effect is verified by experiments:
[0096] (1) in 6-well plate with 5×10 5 The density of granule cells / well was planted into PC9 non-small cell lung cancer cell line respectively, and treated with different concentrations of LB-Pi-A12-TH (0, 0.1, 0.3, 1, 3, 10 μM) for 6 hours, or 1 μM Different time points (0, 1, 3, 6, 12, 24 hours).
[0097] (2) After the cells adhere to the wall, add LB-Pi-A12-TH to start the treatment.
[0098] (3) The total protein was collected from the lysate, and after quantification, the expression levels of total ERK and phosphorylated RSK were detected by Western Blot.
[0099] Result analysis: Test results such as figure 1 As shown in B, by Western Blot analysis, LB-Pi-A12-TH treatment can effectively promote the degradation of ERK1 / 2 and the decrease of RSK phosphorylation level as the concentration increase...
Embodiment 2
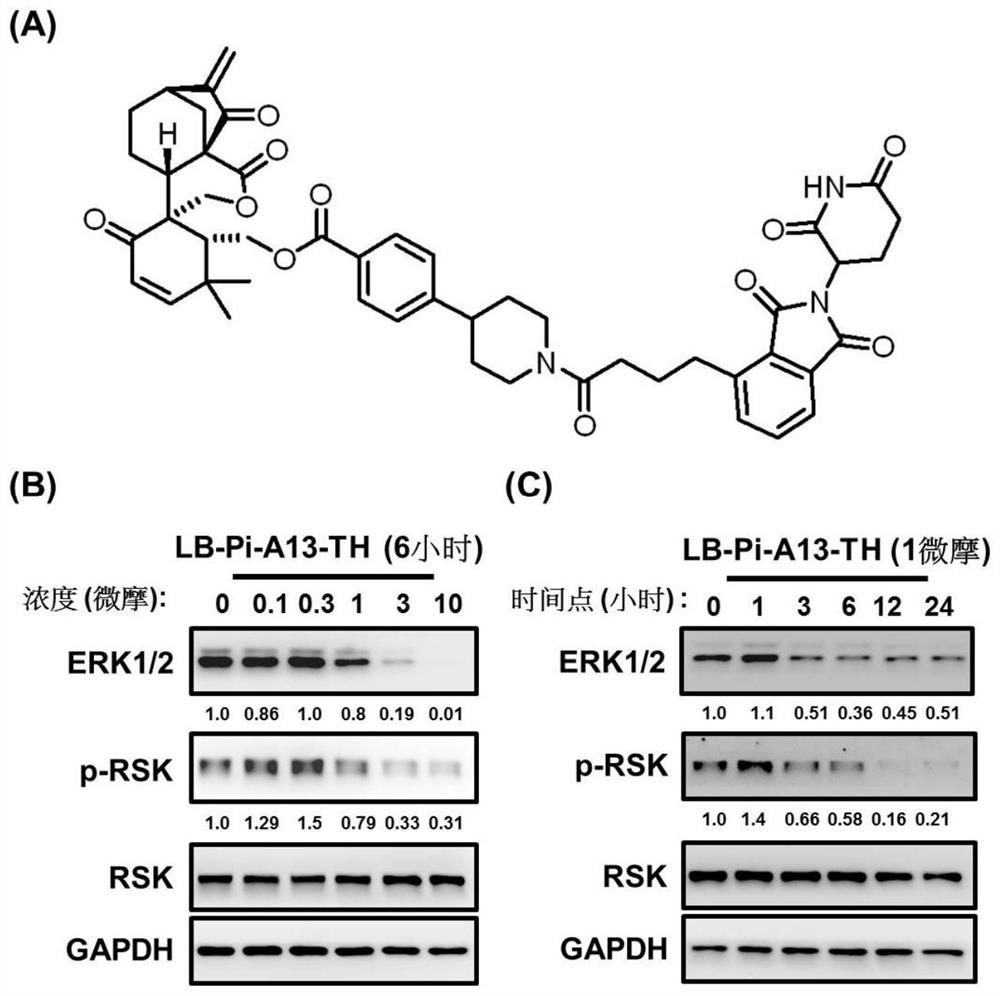
[0101] A targeting chimera (represented by LB-Pi-A13-TH), whose structure is as follows figure 2 As shown in A, its ERK1 / 2 degradation effect is verified by experiments:
[0102] (1) in 6-well plate with 5×10 5 The density of granule cells / well was planted into PC9 non-small cell lung cancer cell line respectively, and treated with different concentrations of LB-Pi-A14-TH (0, 0.1, 0.3, 1, 3, 10 μM) for 6 hours, or 1 μM Different time points (0, 1, 3, 6, 12, 24 hours).
[0103] (2) After the cells adhered to the wall, LB-Pi-A13-TH was added to start the treatment.
[0104] (3) The total protein was collected from the lysate, and after quantification, the expression levels of total ERK and phosphorylated RSK were detected by Western Blot.
[0105] Result analysis: Test results such as figure 2As shown in B, by Western Blot analysis, LB-Pi-A13-TH treatment can effectively promote the degradation of ERK1 / 2 and the decrease of RSK phosphorylation level as the concentration ...
Embodiment 3
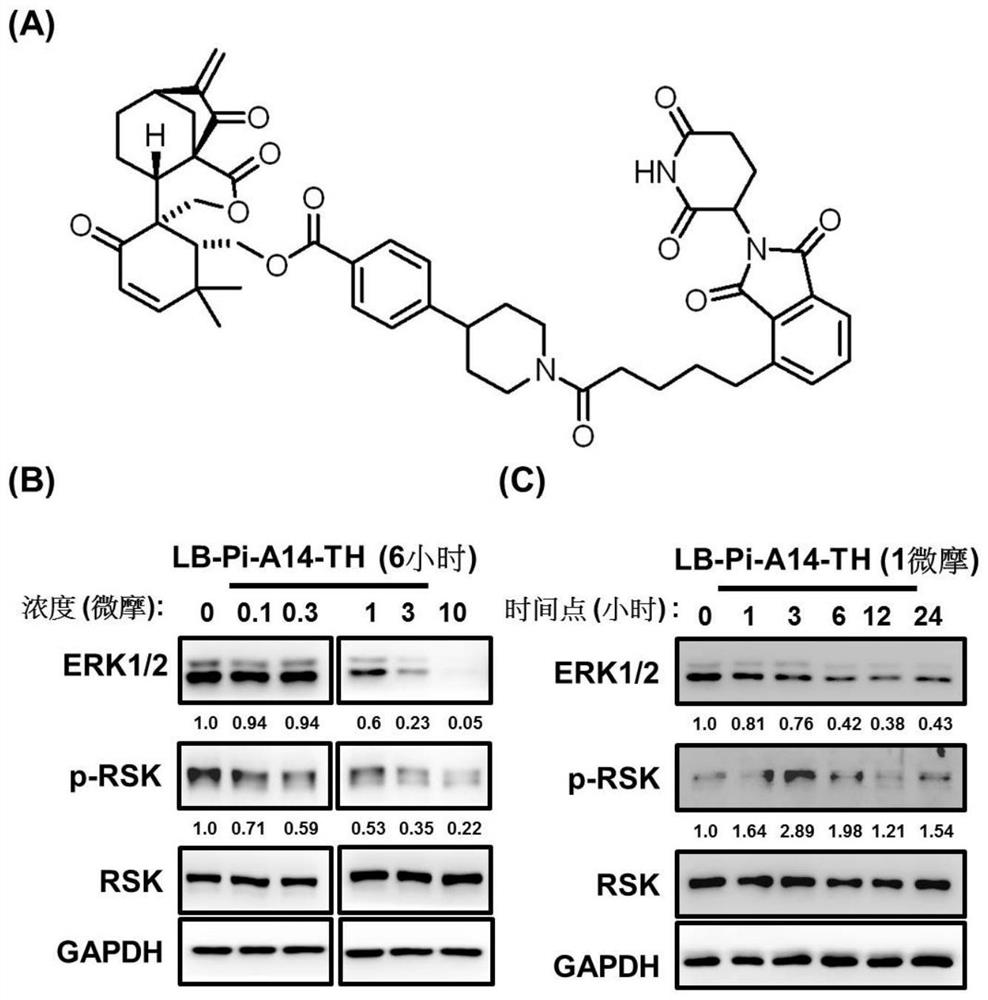
[0107] A targeting chimera (represented by LB-Pi-A14-TH), whose structure is as follows image 3 As shown in A, its ERK1 / 2 degradation effect is verified by experiments:
[0108] (1) in 6-well plate with 5×10 5 The density of granule cells / well was planted into PC9 non-small cell lung cancer cell line respectively, and treated with different concentrations of LB-Pi-A14-TH (0, 0.1, 0.3, 1, 3, 10 μM) for 6 hours, or 1 μM Different time points (0, 1, 3, 6, 12, 24 hours).
[0109] (2) After the cells adhere to the wall, add LB-Pi-A14-TH to start the treatment.
[0110] (3) The total protein was collected from the lysate, and after quantification, the expression levels of total ERK and phosphorylated RSK were detected by Western Blot.
[0111] Result analysis: Test results such as image 3 As shown in B, by Western Blot analysis, LB-Pi-A14-TH treatment can effectively promote the degradation of ERK1 / 2 and the decrease of RSK phosphorylation level as the concentration increase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com