Heterocyclic compounds and their preparation methods and applications
A compound and heterocyclic technology, applied in the field of heterocyclic compounds and their preparation and application, can solve problems such as property differences, and achieve the effect of inhibiting cancer or related diseases, significant cancer or related diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0569] 1 Free base amorphous
[0570] The compound of formula I (compound 45 in the patent) was prepared by referring to the method of Example 49 in Patent No. CN113087700A
[0571] Synthesis of 2-((S)-4-((R)-4-chloro-2'-((tetrahydro-1H-pyrrolazin-7a(5H)-yl)methoxy)-2,3,5' ,8'-Tetrahydro-6'H-spiro[indene-1,7'-quinazolin]-4'-yl)piperazin-2-yl)acetonitrile
[0572] To 2-((S)-4-((R)-4-chloro-2'-((tetrahydro-1H-pyrrolazin-7a(5H)-yl)methoxy) under Ar at 0 °C -2,3,5',8'-Tetrahydro-6'H-spiro[indene-1,7'-quinazolin]-4'-yl)piperazin-2-yl)acetonitrile (1.0 g, 1.88 g mmol), DMAP (0.23 g, 1.88 mmol), TEA i.e. triethylamine (0.57 g, 5.63 mmol) and 2-fluoroacrylic acid (0.51 g, 5.63 mmol) in dichloromethane (DCM for short, 20 mL) join T 3 P is propylphosphoric anhydride (2.39 g, 3.75 mmol) and the mixture was stirred at room temperature for 1 hour. Water was added and the resulting mixture was extracted three times with DCM. The organic layers were combined, washed with brine, washed ...
Embodiment 2
[0612] Example 2 Preparation of hydrochloride crystal form
[0613] 1 Hydrochloride Form A
[0614] The hydrochloride salt crystal form A is dissolved in ethanol (abbreviated as EtOH) / n-heptane (1:4, v / v by the free base amorphous (the compound of formula I obtained by the method in Example 1), and the weight to volume ratio is 40g / L), add hydrochloric acid (acid-base molar ratio 1:1), stir at room temperature for 3 days, remove the supernatant by centrifugation, and dry the obtained solid overnight at room temperature.
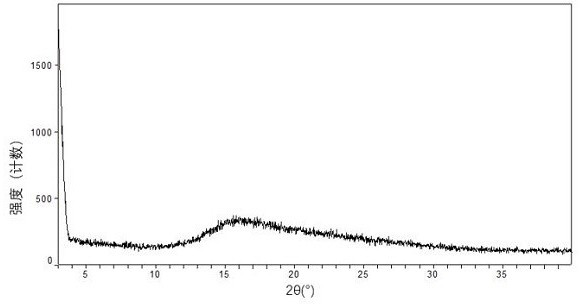
[0615] XRPD characterization results such as Figure 17 As shown, the diffraction data are shown in Table 7 below.
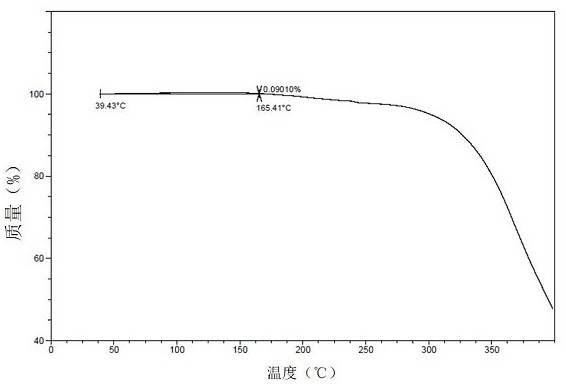
[0616] TGA results ( Figure 18 ) shows that the sample has an 8.93% weight loss before being heated to 160°C at room temperature.
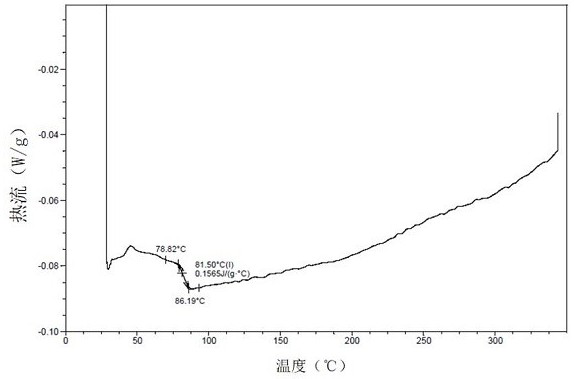
[0617] DSC results ( Figure 19 ) shows that the sample has an endothermic peak at 158.4 °C (peak temperature).
[0618] 1 H-NMR results ( Figure 20 ) shows that the molar ratio of residual EtOH to API in...
Embodiment 3
[0647] Example 3 Preparation of sulfate crystal form
[0648] 1 Sulfate Form A
[0649] Sulfate crystal form A is mixed with acetone / n-heptane (1:1, v / v) by the free base amorphous (the compound of formula I obtained by the method in Example 1), and then sulfuric acid (acid-base feeding molar) is added. Ratio 1:1) After stirring at room temperature for about 3 days, the supernatant was removed by centrifugation, and the obtained solid was dried at room temperature overnight.
[0650] The XRPD results of sulfate crystal form A are as follows Figure 33 As shown, the diffraction data are shown in Table 11 below.
[0651] TGA results ( Figure 34 ) shows a 6.52% weight loss when the sample is heated from room temperature to 150 °C.
[0652] DSC results ( Figure 35 ) showed 2 endothermic peaks at 108.7 and 148.3 °C (peak temperature).
[0653] 1 H-NMR results ( Figure 36 ) shows that the molar ratio of residual n-heptane to API in the sample is 0.05 (corresponding to a w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com