Application of RDH10 and/or prodrug thereof in preparation of medicine for treating and/or preventing diabetic myocardial injury
A technology of RDH10, 1.RDH10, applied in the field of gene therapy, to achieve the effect of preventing/treating diabetic myocardial injury, inhibiting cardiac dysfunction and myocardial structural remodeling, and protecting cardiac function and myocardial structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
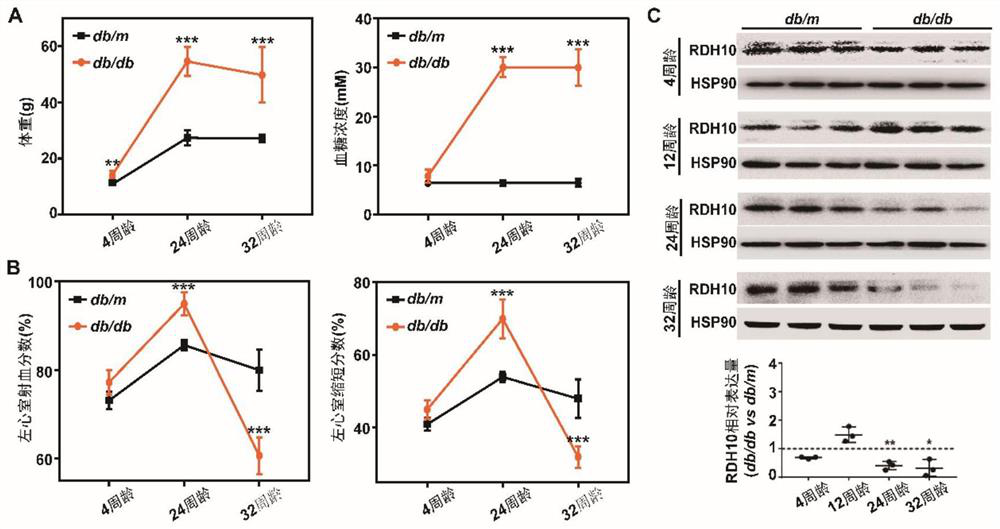
[0029] Example 1 Effect of diabetic myocardial injury on the expression level of myocardial RDH10 gene
[0030] In order to verify whether diabetic myocardial injury has an effect on the expression level of myocardial RDH10 gene, type 2 diabetes model db / db mice and their littermate control db / m mice (purchased from Jiangsu Jicui Yaokang) each three, fasted for 12 hours (without water) to measure their body weight and blood sugar, and then use small animal echocardiography to detect their heart function, and finally take their myocardial tissue and extract myocardial total protein to detect RDH10 The expression is as follows:
[0031] (1) Detection of body weight and blood sugar:
[0032] 4. The body weight and blood glucose test results of mice aged 24 and 32 weeks were as follows: figure 1 As shown in A, it can be seen that the blood glucose of db / db mice at the age of 4 weeks is equal to that of db / m mice, but their body weight is higher than that of db / m mice; while the ...
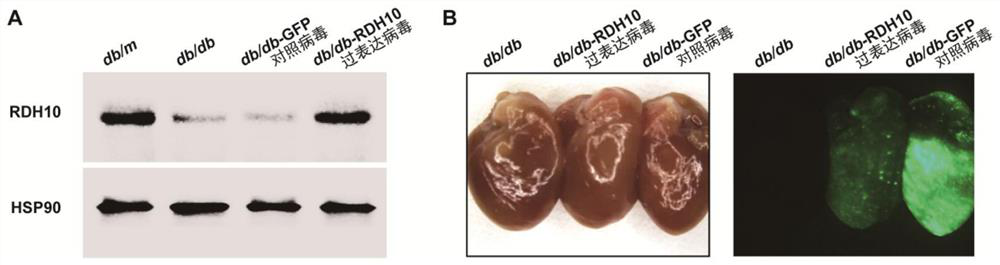
Embodiment 2
[0045] Example 2 Protection of RDH10 on cardiac function in mice with diabetic myocardial injury
[0046] 1. Experiment preparation
[0047] (1) Experimental materials:
[0048] 1) 30 type 2 diabetes model db / db mice at the 8th week after the blood sugar stably increased, and 10 db / m mice at the 8th week after the stably increased blood sugar in the same littermate (purchased from Jiangsu Jizhuyao Kang);
[0049] 2) Recombinant RDH10 overexpression type 9 adeno-associated virus, recombinant GFP control type 9 adeno-associated virus (purchased from Shanghai Hanheng Biotechnology Co., Ltd.);
[0050] Among them, the 16-week-old mice were defined as the 8th week after the blood glucose level increased steadily.
[0051] (2) Experimental grouping:
[0052] Thirty type 2 diabetes model db / db mice were randomly divided into three groups: 1) through the tail vein injection of recombinant RDH10 overexpressing adeno-associated virus type 9 (the virus injection volume was 0.8×10 11...
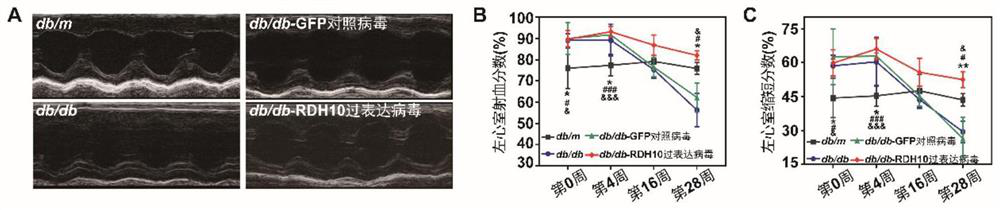
Embodiment 3
[0062] Example 3 Protection of RDH10 on myocardial structure in diabetic myocardial injury mice
[0063] (1) Preparation of heart paraffin sections:
[0064] After the remaining mice in Example 2 reached the end point of the experiment (28 weeks of stable increase in blood sugar), they were fasted 12 hours in advance without food or water. After fasting, the mouse was anesthetized with pentobarbital, the chest was opened with ophthalmological scissors, the right atrial appendage was cut open, and sterile PBS was perfused from the left ventricle of the mouse with a syringe until the blood flowed out. Finally, cut out the heart, rinse with PBS until there is no blood stain, and fix with 4% paraformaldehyde for 16h. The next day, wash with PBS 3 times, 30 minutes each time; 25% ethanol, 50% ethanol, 75% ethanol, 90% ethanol, absolute ethanol (I), absolute ethanol (II) gradient dehydration, each 30 minutes; Toluene (I) and xylene (II) each for 15 minutes; paraffin (I) and paraff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com