Preparation method of iso-tridecanol
A technology of isotridecanol and ionic liquid, applied in the field of preparation of isotridecanol, can solve the problems such as the inability to solve the separation and deactivation of homogeneous rhodium, the inability of large-scale application of rhodium catalyst, and the inability to obtain a hydrogenation process. , to achieve good industrialization prospects, good stability and good recyclability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
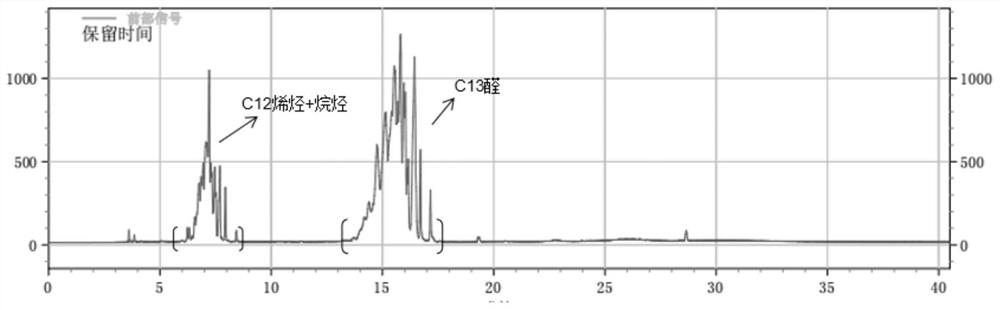
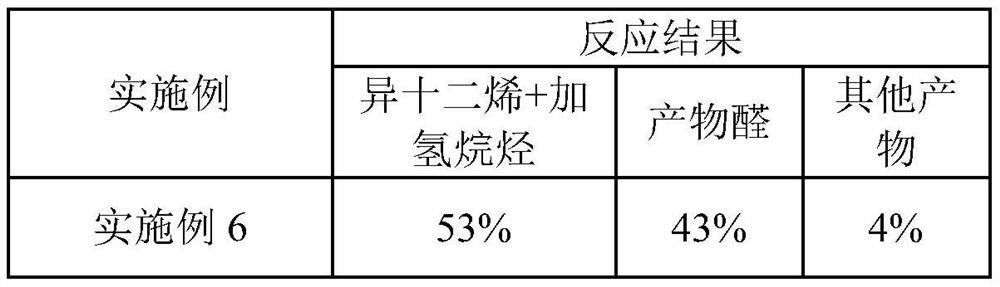
[0041] Add 70g of 1-butyl-3-methylimidazolium hexafluorophosphate, 45mL of n-heptane, 35mL of isododecene, 2.1g of phosphine ligand L1 and Rh(CO) into a 250mL autoclave. 2After acac 40 mg, the reaction kettle replaced the air with Ar three times, and then after vacuuming, the synthesis gas (CO:H 2 =1:1, volume ratio) to 4MPa, start stirring, and start to heat up. The temperature in the kettle rose to 120° C., and the pressure was 5.5 MPa. The reaction started, and the reaction pressure was controlled to maintain at 5 MPa, and the reaction time was 6 hours. After the reaction was completed, it was lowered to room temperature, the pressure was released to normal pressure, and the kettle was opened. The reaction solution in the kettle was separated into phases, and after the upper organic phase was separated, the lower ionic liquid phase was extracted twice with 50 mL of n-heptane. After combining the upper organic phases, a reaction liquid containing isomeric tridecanol was ob...
Embodiment 2
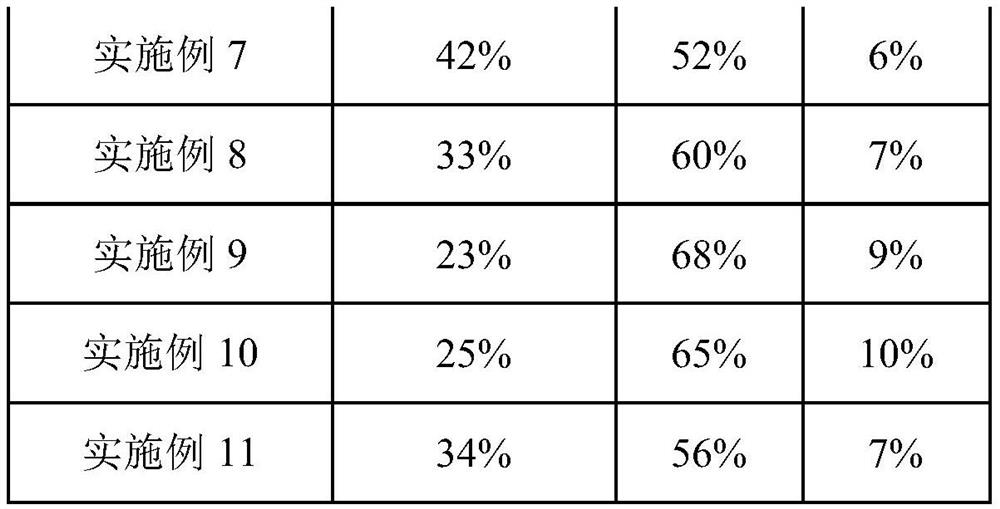
[0043] After adding 70g of 1-hexyl-3-methylimidazolium hexafluorophosphate, 45mL of methylcyclohexane, 35mL of isododecene, 4.4g of phosphine ligand L2 and 125mg of rhodium octanoate into a 250mL autoclave, the reaction kettle was Ar replaced the air three times, and then after vacuuming, the synthesis gas (CO:H 2 =1:1, volume ratio) to 4MPa, start stirring, and start to heat up. The temperature in the kettle rose to 120° C., and the pressure was 5.5 MPa. The reaction started, and the reaction pressure was controlled to maintain at 5 MPa, and the reaction time was 6 hours. After the reaction was completed, it was lowered to room temperature, the pressure was released to normal pressure, and the kettle was opened. The reaction liquid in the kettle was separated into phases, and after the upper organic phase was separated, the lower ionic liquid phase was extracted twice with 50 mL of methylcyclohexane. After combining the upper organic phases, a reaction liquid containing iso...
Embodiment 3
[0045] After adding 70g of 1-hexyl-3-methylimidazolium hexafluorophosphate, 45mL of dodecane, 35mL of isododecene, 5.6g of phosphine ligand L3 and 40mg of rhodium acetylacetonate dicarbonyl into a 250mL autoclave, the reaction kettle The air was replaced with Ar three times, and after vacuuming, synthesis gas (CO:H 2 =1:1, volume ratio) to 4MPa, start stirring, and start to heat up. The temperature in the kettle rose to 120° C., and the pressure was 5.5 MPa. The reaction started, and the reaction pressure was controlled to maintain at 5 MPa, and the reaction time was 6 hours. After the reaction was completed, it was lowered to room temperature, the pressure was released to normal pressure, and the kettle was opened. The reaction liquid in the kettle was separated into phases, and after the upper organic phase was separated, the lower ionic liquid phase was extracted twice with 50 mL of dodecane respectively. After combining the upper organic phases, a reaction liquid contain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com