Synthesis method of polycyclic norbornene derivative
A technology for norbornene and a synthesis method, applied in the field of CO7C, can solve the problems of difficulty in suppressing oligomers such as trimerization and tetramerization, increase separation and purification cost and energy consumption, and difficulty in suppressing by-products, etc., and achieve excellent chemical resistance. excellent optical properties, moisture resistance, and high recycling rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
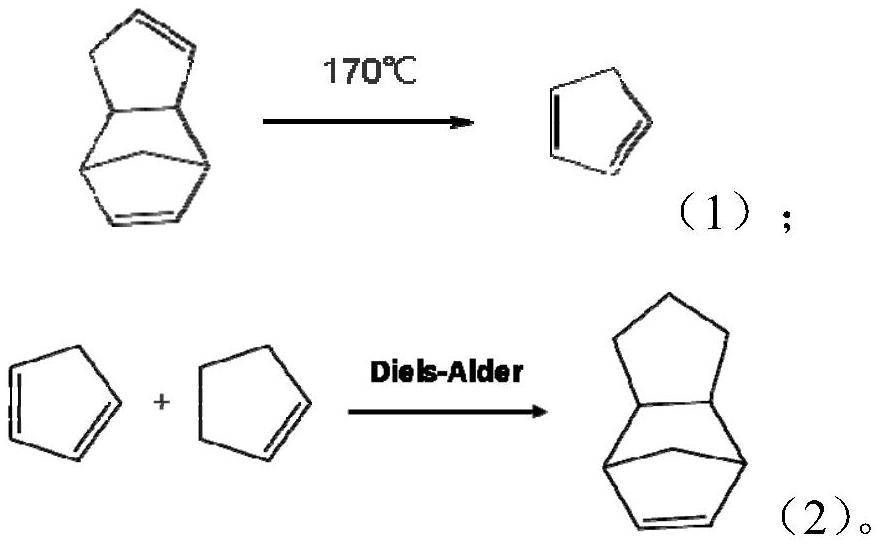
Image
Examples
Embodiment 1
[0030] After debugging the temperature control systems, instruments and valves of the device, replace the reactor with nitrogen and check the device for leaks. Use a metering pump to add cyclopentene and dicyclopentadiene in a molar ratio of 2.5:1 to the reactor, and inject 1.5MPa high-purity nitrogen into the reactor. Set the reactor temperature to 250 °C. After the temperature reaches the set temperature, the pressure in the reactor reaches 4.8 MPa. Under this condition, stop heating after 1 hour of reaction, and pass water to the cooling coil to cool down to stop the reaction. The crude product is taken out, and the crude product is rectified and separated, and the obtained cyclopentene light components and non-target heavy components are returned to the reactor for recycling, and the target product yield is recorded as 66.2% (the weight of the target product accounts for the added The mass percent of the total weight of cycloolefins and dicyclopentadiene). The purity of ...
Embodiment 2
[0034] After debugging the temperature control systems, instruments and valves of the device, replace the reactor with nitrogen and check the device for leaks. Use a metering pump to add 1-methylcyclopentene and dicyclopentadiene to the reactor at a molar ratio of 2:1, and inject a mixture of nitrogen and hydrogen with a hydrogen volume content of 50% into the reactor to 1.5 MPa. Set the reactor temperature to 260 °C. After the temperature reaches the set temperature, the pressure in the reactor reaches 4.9 MPa. Under this condition, stop heating after 1.5 hours of reaction, and pass water to the cooling coil to cool down to stop the reaction. The crude product was taken out, and the crude product was separated by rectification, and the obtained 1-methylcyclopentene light component and non-target heavy component were returned to the reactor for recycling, and the yield of the target product was measured to be 51.5%. The purity of the target product obtained through rectificat...
Embodiment 3
[0038] After debugging the temperature control systems, instruments and valves of the device, replace the reactor with nitrogen and check the device for leaks. Use a metering pump to add cyclohexene and dicyclopentadiene in a molar ratio of 3:1 to the reactor, and inject a hydrogen-nitrogen mixture with a hydrogen volume content of 60% to 1.5 MPa into the reactor. Set the reactor temperature to 190 °C. After the temperature reaches the set temperature, the pressure in the reactor reaches 3.1 MPa. Under this condition, stop heating after 1 hour of reaction, and pass water to the cooling coil to cool down to stop the reaction. The crude product was taken out, and the crude product was rectified and separated, and the obtained cyclohexene light component and non-target heavy component were returned to the reactor for recycling, and the yield of the target product was measured to be 55.5%. The purity of the target product obtained through rectification as measured by gas chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com