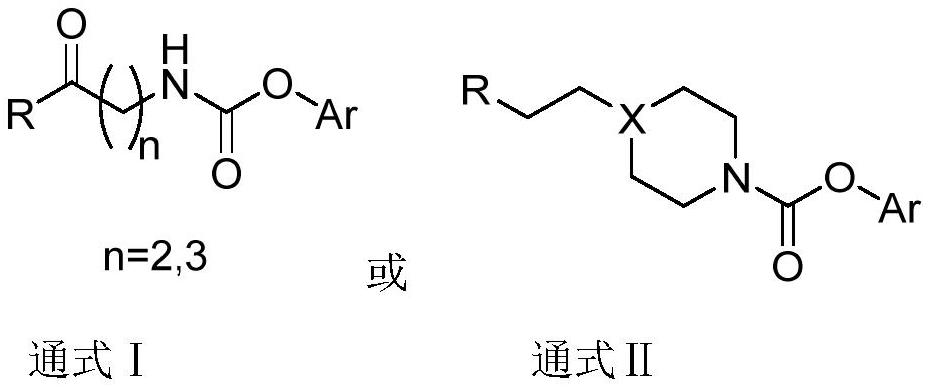
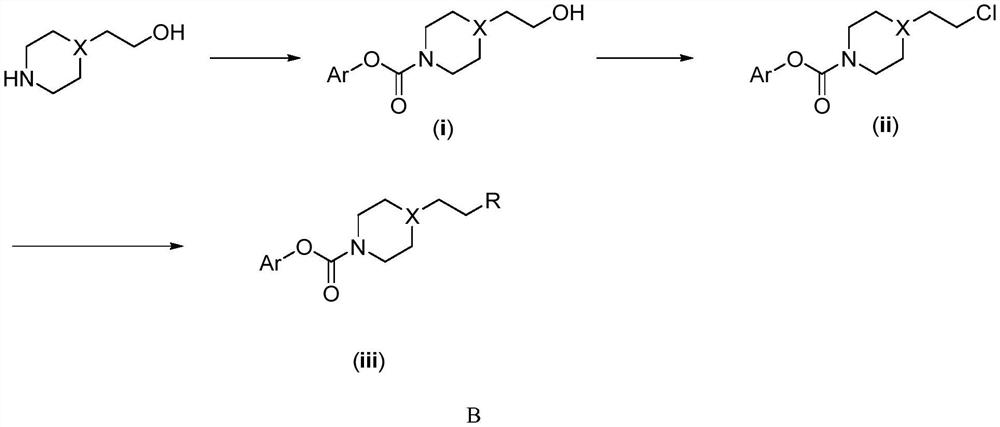
Carbamate TRPV1 antagonism/FAAH inhibition double-target drug, and preparation method and application of carbamate TRPV1 antagonism/FAAH inhibition double-target drug
A technology of carbamates and carbamates, applied in the fields of carbamate TRPV1 antagonistic/FAAH inhibitory dual-target drugs and their preparation and application, can solve problems such as elevated body temperature, eliminate side effects and reduce adverse reactions , the effect of increasing the therapeutic window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
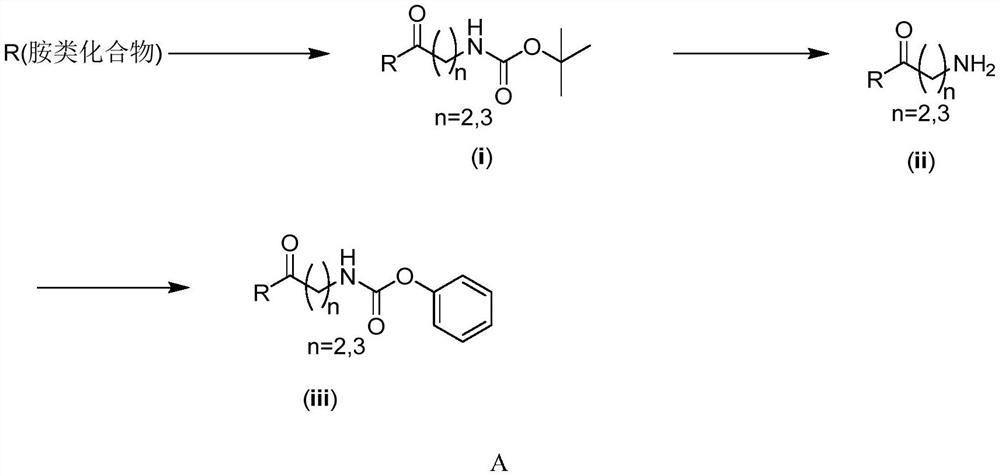
[0048] Embodiment 1: Synthesis of phenyl (4-(4-(2-(4-methylpiperidin-1-yl) benzyl) piperazin-1-yl)-4-oxobutyl) carbamate
[0049]
[0050] (1) Synthesis of tert-butyl (4-(4-(2-(4-methylpiperidin-1-yl) benzyl) piperazin-1-yl)-4-oxobutyl) carbamate
[0051] At room temperature, Boc-4-aminobutyric acid (2.50 mmol), 1-hydroxybenzotriazole monohydrate (HOBt·H 2 O) (3.125mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (3.125mmol), triethylamine (7.50mmol), then add 10mL di Chloromethane was used as a solvent, and after stirring at room temperature for 0.5h, 1-(2-(4-methylpiperidin-1-yl)benzyl)piperazine (2.50mmol) was added, and the reaction was continued for 8h at room temperature. After the reaction was completed, the reaction solution was successively washed with 10% citric acid solution (10mL×3), saturated saline solution (10mL×3), dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain tert-butyl (4-(4-(2...
Embodiment 2
[0057] Example 2: Synthesis of phenyl-(3-oxo-3-(2-(4-phenylthiazol-2-yl)pyrrolidin-1-yl)propyl)carbamate
[0058]
[0059] Replace 1-(2-(4-methylpiperidin-1-yl) benzyl) piperazine in Example 1 with (S)-4-phenyl-2-(pyrrolidin-2-yl)thiazole, Boc-4-aminobutyric acid in Example 1 was replaced with Boc-β-alanine, and other references were made to the preparation method in Example 1 to obtain Compound 2, a pale yellow oil with a yield of 76.6%. The experimental data are as follows:
[0060] C 23 h 23 N 3 o 3 S, light yellow oil, (76.6%, yield), 1 H NMR (CDCl 3 ,300MHz)δppm7.92(m,2H,Ar-H),7.48-7.33(m,7H,Ar-H and thiazole),7.24-7.13(m,3H,Ar-H andNH),5.69-5.24(m ,1H,pyrrolidine),3.90-3.65(m,2H,pyrrolidine),3.63-3.46(m,2H,CH 2 ),2.77-2.59(m,2H,CH 2 ), 2.51-2.31 (m, 2H, pyrrolidine), 2.29 (s, 2H, pyrrolidine).
Embodiment 3
[0061] Example 3: Synthesis of phenyl (4-(4-chloropyrimidin-2-yl) piperazin-1-yl)-4-oxobutyl) carbamate
[0062]
[0063] Replace 1-(2-(4-methylpiperidin-1-yl)benzyl)piperazine in Example 1 with 4-chloro-2-(piperazin-1-yl)pyrimidine to obtain compound 3, white Paste, yield 63.3%. The experimental data are as follows:
[0064] C 17 h 18 ClN 5 o 3 ,white paste,(63.3%,yield), 1 H NMR (DMSO, 300MHz) δppm 8.11 (d, J = 6.2Hz, 1H, Ar-H), 7.83-7.73 (t, 1H, NH), 7.37 (t, J = 7.8Hz, 2H, Ar-H) ,7.19(t,J=7.4Hz,1H,Ar-H),7.14-7.06(m,2H,Ar-H),6.85(d,J=6.2Hz,1H,Ar-H),3.70-3.51( m,8H,piperazine),3.11(q,J=6.6Hz,2H,CH 2 ),2.41(q,J=8.2,7.8Hz,2H,CH 2 ), 1.73(t, J=7.2Hz, 2H, CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com