Method for terminating anionic polymerization reaction
An anionic polymerization and reaction technology, applied in the field of terminators, can solve the problems of terminator residues, alkali metal compound residues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
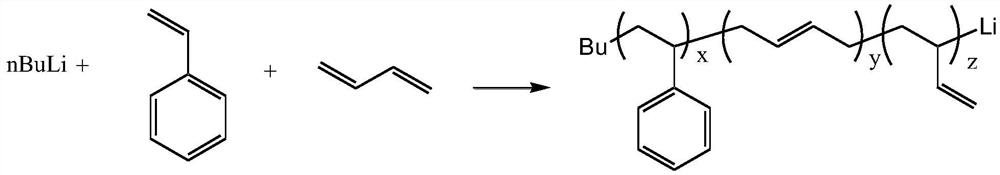
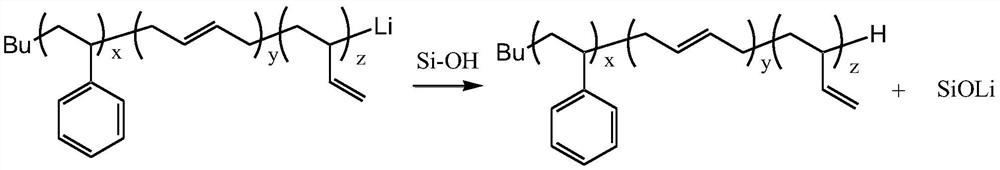
[0043] Cyclohexane (100 mL) dehydrated was used as the reaction solvent and styrene (6 g) and butadiene (4 g) were used as comonomers, and the temperature was raised to 60° C. after stirring evenly. Add n-butyllithium (0.01g) as an initiator to carry out anionic polymerization reaction of styrene-butadiene copolymer. After reacting for 1 hour, cool down and take a sample to observe that the polymerization reaction liquid is orange-red, indicating that the polymer still maintains activity. After the active polymer solution was sampled to remove the solvent, it was dried in an oven at 100° C. for 2 hours, and its lithium metal concentration (122 ppm) was analyzed by an inductively coupled plasma atomic emission spectrometer (ICP-OES). Lignin (alkali lignin, purchased from Sigma-Aldrich's Lignin, alkali) is used as a terminator and metal remover for anionic polymerization and added to the active polymer solution, wherein the active polymer and lignin weight ratio is 100:5 , where...
Embodiment 2
[0045] Cyclohexane (100 mL) dehydrated was used as the reaction solvent and styrene (6 g) and butadiene (4 g) were used as comonomers, and the temperature was raised to 60° C. after stirring evenly. Add n-butyllithium (0.01 g) as an initiator to carry out anionic polymerization of styrene-butadiene copolymer. After reacting for 1 hour, the temperature was lowered and samples were taken to observe that the polymerization reaction solution was orange-red, indicating that the polymer still remained active. After the active polymer solution was sampled to remove the solvent, it was dried in an oven at 100° C. for 2 hours, and its lithium metal concentration (122 ppm) was analyzed by an inductively coupled plasma atomic emission spectrometer (ICP-OES). Diatomaceous earth (purchased from Sigma-Aldrich 545) as the terminator of anionic polymerization and metal removal agent and adding the solution of active polymer, wherein active polymer and diatomite weight ratio is 100:5, and wh...
Embodiment 3
[0047] Cyclohexane (100 mL) dehydrated was used as the reaction solvent and styrene (6 g) and butadiene (4 g) were used as comonomers, and the temperature was raised to 60° C. after stirring evenly. Add n-butyllithium (0.01g) as an initiator to carry out anionic polymerization reaction of styrene-butadiene copolymer. After reacting for 1 hour, cool down and take a sample to observe that the polymerization reaction liquid is orange-red, indicating that the polymer still maintains activity. After the active polymer solution was sampled to remove the solvent, it was dried in an oven at 100° C. for 2 hours, and its lithium metal concentration (122 ppm) was analyzed by an inductively coupled plasma atomic emission spectrometer (ICP-OES). Silica gel (Silica gel purchased from Sigma-Aldrich, particle size: 70 ~ 230mesh) is used as a terminator of anionic polymerization reaction and a metal remover and added to the solution of active polymer, wherein the weight ratio of active polymer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com