Immunostimulatory micelle compositions
A composition, micellar technology, applied in the field of treatment of diseases and disorders such as cancer, which can solve the problems of accelerating blood clearance of antibodies and destroying the effectiveness of therapeutic nanoparticle delivery systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0361] Example 1: Preparation of micelles and liposomes
[0362] Micelles were made from 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) obtained from Lipoid GmbH. Briefly, lipids were dissolved in tert-butanol:water (9:1 by volume) to a final concentration of 5-10 mM in glass vials and placed under magnetic stirring and heated to 50°C until completely dissolved . Solvent was removed by freezing the vial in liquid nitrogen followed by lyophilization overnight. Initial contact between the lipid and solvent was established by dispersing the dried lipid in a buffer solution containing 150 mM NaCl, 10 mM phosphate (pH = 7.4), exposing the vial to gentle vortexing, followed by exposure to sonication Micelles were prepared for 30 minutes to ensure the formation of micellar structures. The dispersion was vortexed again, and then the dispersion was exposed to further sonication for 30 minutes. Store micelles at 4 °C until use an...
Embodiment 2
[0364] Example 2: Preparation of micelles incorporating toll-like receptor 7 (TLR7) agonists
[0365] 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) obtained from Lipoid GmbH and TLR7 agonist 1v270 ( C57H93N6O12P, Mw=1085.4, (2,3-bis(oleoyloxy)propyl phosphate 2-(4-((6-amino-2-(2-methoxyethoxy)-8-oxo- 7H-purin-9(8H)-yl)methyl)benzamido)ethyl ester) into micelles. The chemical structure of 1v270 is outlined in Figure 2. Briefly, lipids were dissolved in tert-butanol: In water (volume ratio 9:1), reach a final concentration of 5-10 mM (DSPE-PEG2000) or 1-3 mM (1v270) in a glass vial, and place under magnetic stirring and heat to 50 degrees Celsius until completely dissolved. The two lipid dispersions were then mixed to the desired ratio (95:5 to 80:20 DSPE-PEG2000:1v270 molar ratio). The solvent was removed by freezing the vial in liquid nitrogen and then lyophilized overnight. Lipids were dispersed in a buffer solution con...
Embodiment 3
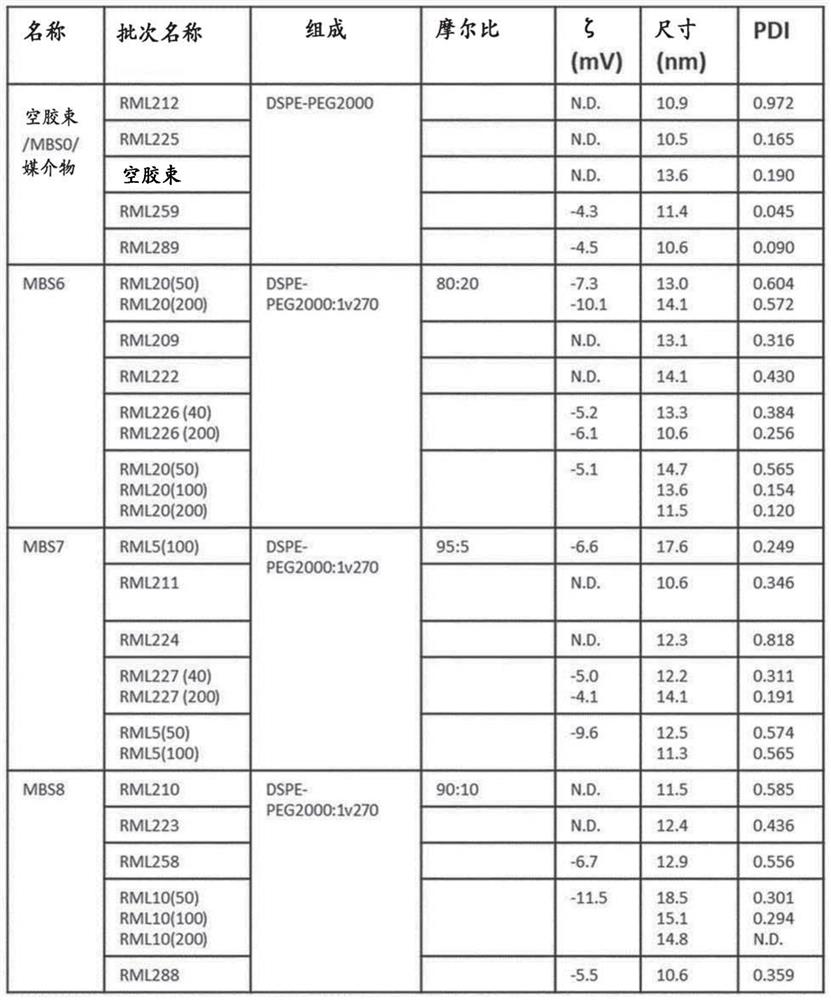
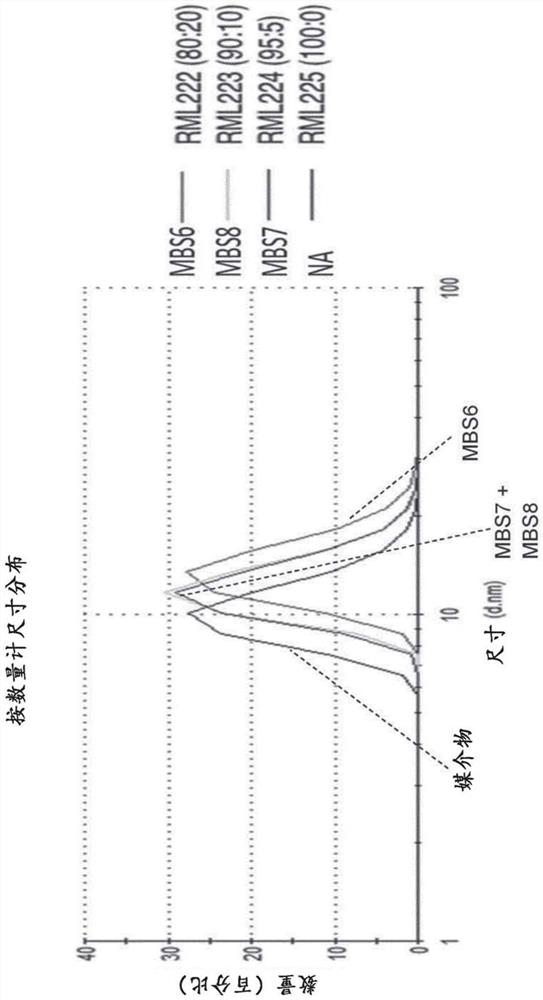
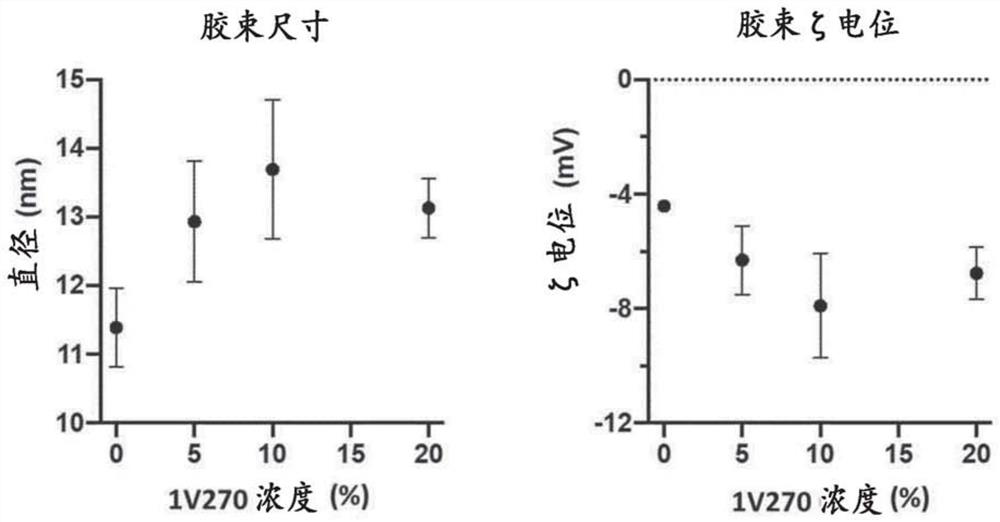
[0366] Example 3: Characterization of composition-dependent micelle size and surface charge
[0367] Micelles were prepared as outlined in Figure 1 in an attempt to prepare stable formulations of the TLR7 agonist 1v207 in aqueous solvents allowing injection in saline buffer. Micelles were prepared as described in Example 1+2, and prepared by 5% (w / w) glucose, 10mM HEPES, 1mM CaCl 2 The size (diameter) is measured in nanometers (nm) by dynamic light scattering in a buffer consisting of MilliQ water pH 7.4. The micelles without 1V207 had an average size (according to particle distribution by number) of 11.5 nm, while the micelles containing 1V270 had a particle size ranging from 11.5 nm to 13-14 nm when the 1V270 content was increased from 5% to 20%. Average size (according to distribution by number of particles) (Figures 1B and 1C). with CaCl 2 The zeta potential of empty micelles, measured in glucose buffer in , was about -4 mV, but became more negative when the anionic 1V2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap