Transaminase mutant and application thereof
A kind of mutant, transaminase technology, applied in transaminase mutant and its application field, can solve problems such as unfavorable reaction balance, enzyme instability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0078] According to an exemplary embodiment of the present invention, a recombinant plasmid is provided. The recombinant plasmid contains any of the above DNA molecules. The DNA molecules in the above-mentioned recombinant plasmids are placed in appropriate positions of the recombinant plasmids, so that the above-mentioned DNA molecules can be replicated, transcribed or expressed correctly and smoothly.
[0079] Although the term "containing" is used in the present invention to define the above-mentioned DNA molecule, it does not mean that other sequences irrelevant to its function can be arbitrarily added to both ends of the DNA sequence. Those skilled in the art know that in order to meet the requirements of the recombination operation, it is necessary to add suitable restriction endonuclease enzyme cleavage sites at both ends of the DNA sequence, or additionally add a start codon, a stop codon, etc. Therefore, if using Closed formulations to qualify will not truly cover th...
Embodiment 1
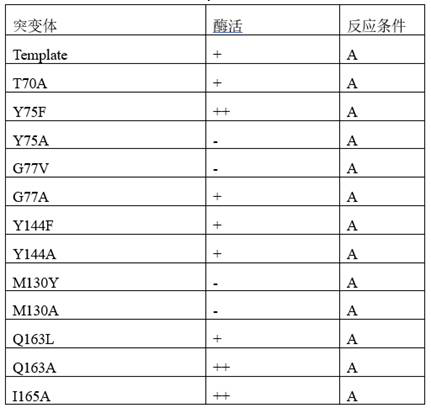
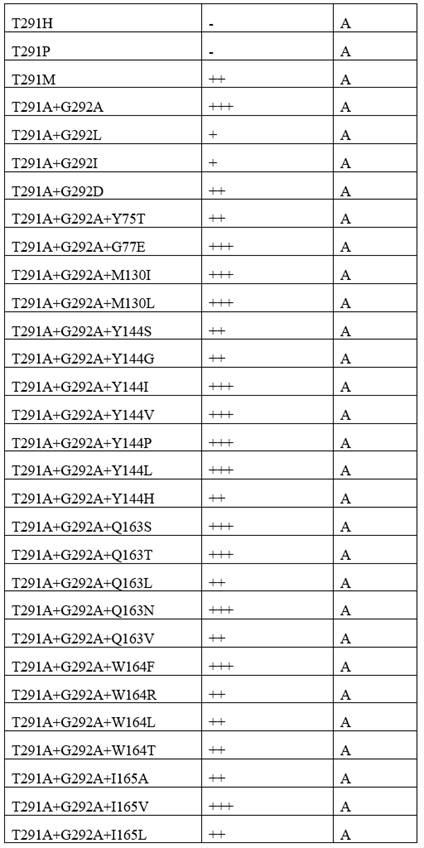
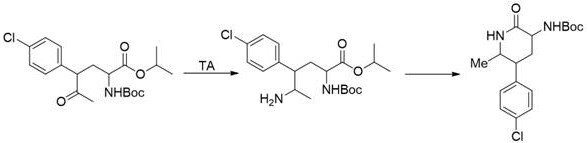
[0090] Template and its mutants catalyze the formation of chiral amines from ketone compounds. The activity was tested under reaction condition M. The results showed that Template had poor catalytic activity to the substrate, while the Template mutant had better catalytic activity, and the mutant T291A+ G292A+ The ee value of I160G+S204L+V244S+T134I+M326Q+Y144W+G77T+I165L+A292S+S278R +Y69H+T70G+G160T+T106S-12aa transaminase mutant increased to more than 90%. After the transformation of the present invention, the template mutants have obtained the catalytic activity for the substrate with larger steric hindrance, the activity of some mutants is greatly improved, and the stereoselectivity of the mutants is also greatly improved, expanding the substrate spectrum . The reaction formula is as follows, and the results are shown in Table 4.
[0091]
[0092] Table 4
[0093]
[0094]
[0095] Remark:
[0096] 1) Reaction conditions M: 0.3 wt enzyme, PLP 1 g / L, DMSO 40%, I...
Embodiment 2
[0100] Template and its mutants catalyze the formation of chiral amines from ketone compounds. The activity was tested under the reaction condition N. The results showed that Template had no catalytic activity to the substrate, while the Template mutant had better catalytic activity. After the modification of the present invention, Template mutants expand the spectrum of catalytic substrates. The reaction formula is as follows, and the results are shown in Table 5.
[0101]
[0102] table 5
[0103]
[0104]
[0105] Remark:
[0106] Reaction conditions M: 1 wt enzyme, PLP 1 g / L, DMSO 20%, IPN 10 eq, 100 V, 0.2 M boric acid pH=10.5, 50 °C, 16 h;
[0107] N.D. means no product generation detected, - means 0~10%, + means 10~20%, ++ means 20~50%, +++ means 50~80%, ++++ means 80~90%, + ++++ means 90%~95%, ++++++ means >95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com