Eye soothing smearing liquid and preparation method thereof
A technology for smearing liquid and eyes, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve eye inflammation, difficulty in resisting foreign bacteria, and human discomfort when using eye drops and other problems, to achieve the effect of fast absorption, repair of microcirculation, and obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0077] Embodiment 1A: low concentration atropine (0.1%)
[0078] An eye soothing smear solution, which is made by mixing phase A that can regulate eye microcirculation, intraocular pressure and relieve visual fatigue, phase B that can supplement eye nutrition, additives and purified water, wherein:
[0079] Phase A is by mass, including 0.1g of atropine;
[0080] Phase B includes the following components by mass: 1g vitamin A, 0.5g vitamin B1, 0.005g vitamin B2, 0.001g vitamin B12, 0.5g adenosine triphosphate, 1g bilberry seed oil and 0.001g lutein;
[0081] The auxiliary agent includes the following components in terms of mass: sodium hyaluronate 0.1g, exopolysaccharide 0.1g, hydrogenated castor oil 6g, cetyl ethylhexanoate 1g and phenoxyethanol 0.6g.
[0082] Purified water 89.093g.
[0083] The above components are divided into oil phase, water phase and added phase, as follows:
[0084] Water phase: 89.093g of purified water, 0.1g of atropine, 0.5g of vitamin B1, 0.005g...
Embodiment 1B
[0089] Embodiment 1B: high concentration atropine (2%)
[0090] Concrete components and preparation method of atropine smear solution (high concentration 2%) are consistent with above-mentioned embodiment 1A, just the concentration of atropine is high concentration, and the consumption of atropine is 2% (being 2g in the aqueous phase). Finally, a yellow liquid can be prepared.
[0091] Stability yields results such as Figure 8 .
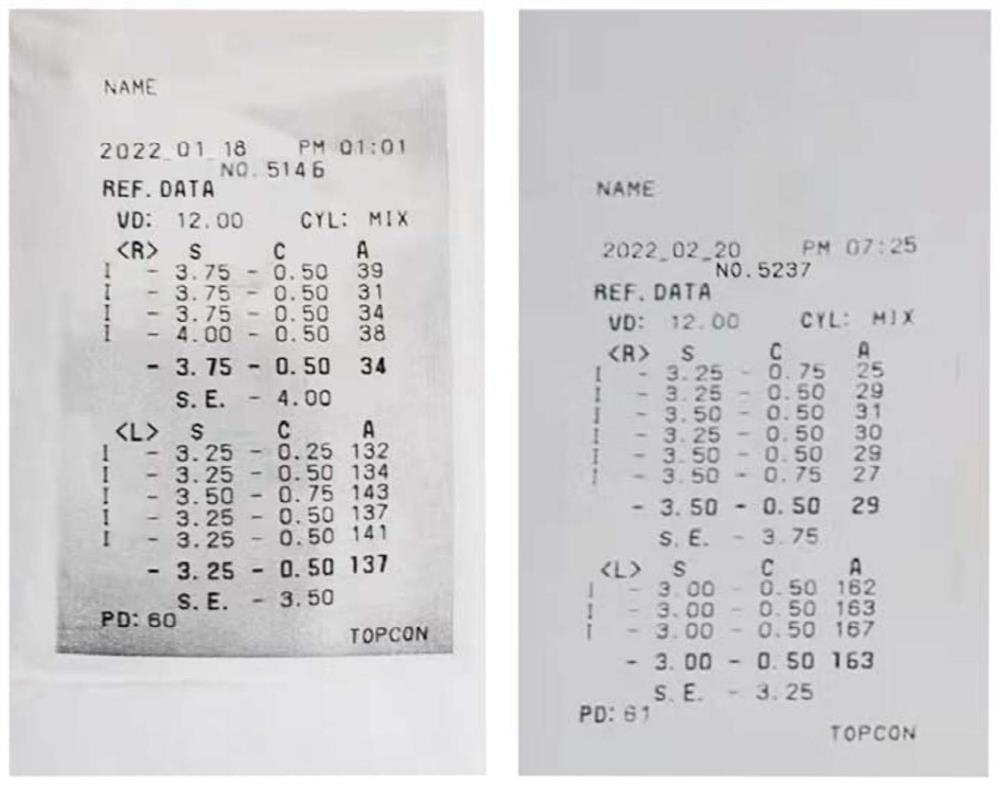
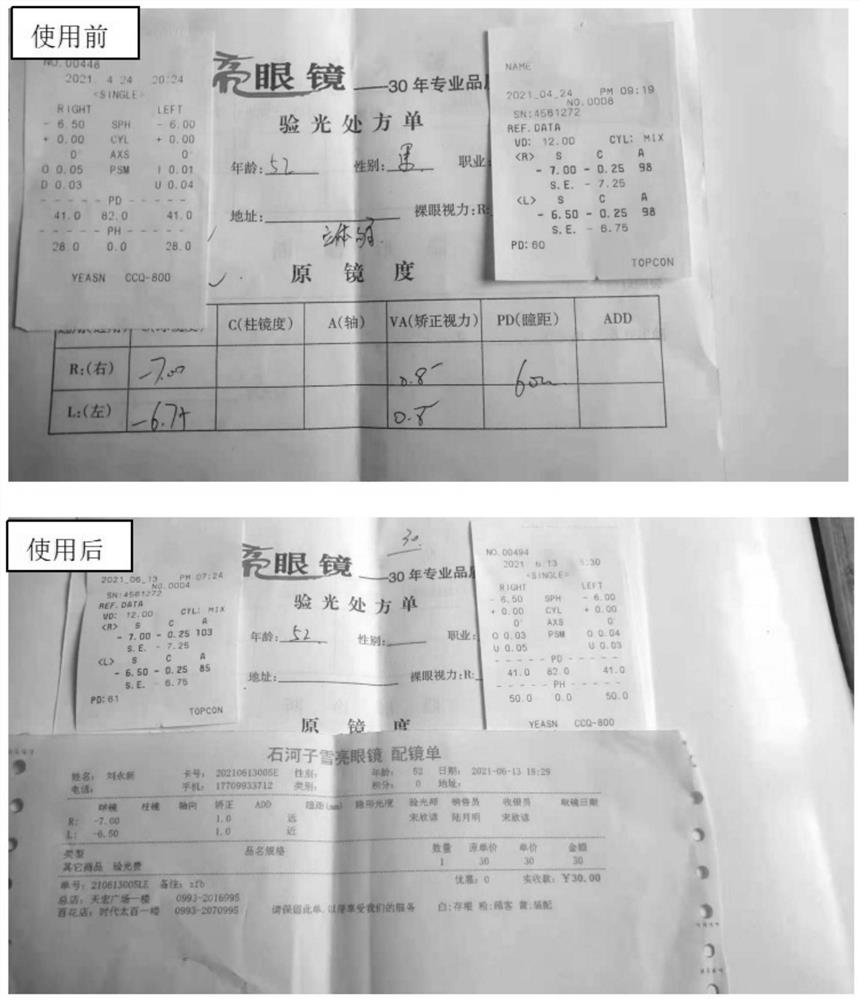
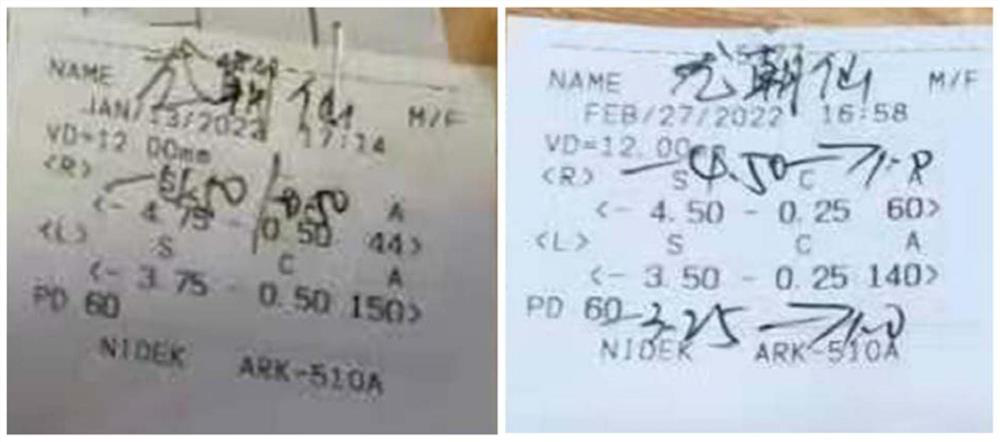
[0092] Low-concentration atropine smear solution has obvious effect on relieving visual fatigue, but not on myopia; high-concentration atropine smear solution has obvious effect on relieving visual fatigue, but has obvious effect on myopia. After using the smear solution for about 4 weeks, the diopter of the left eye dropped from 350 degrees to 325 degrees degrees, down 25 degrees, right eye degree from 400 degrees to 375 degrees, down 25 degrees (see figure 1 ).
Embodiment 2A
[0093] Embodiment 2A: low concentration homatropine (0.1%)
[0094] An eye soothing smear solution, which is made by mixing phase A that can regulate eye microcirculation, intraocular pressure and relieve visual fatigue, phase B that can supplement eye nutrition, additives and purified water, wherein:
[0095] Phase A is by mass, including 0.1 g of homatropine;
[0096] Phase B includes the following components by mass: 1g vitamin A, 0.5g vitamin B1, 0.005g vitamin B2, 0.001g vitamin B12, 0.5g adenosine triphosphate, 1g bilberry seed oil and 0.001g lutein;
[0097] The auxiliary agent includes the following components in terms of mass: sodium hyaluronate 0.1g, exopolysaccharide 0.1g, hydrogenated castor oil 6g, cetyl ethylhexanoate 1g and phenoxyethanol 0.6g.
[0098] Purified water 89.093g.
[0099] The above components are divided into oil phase, water phase and added phase, as follows:
[0100] Water phase: 89.093g purified water, 0.1g homatropine, 0.5g vitamin B1, 0.005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com