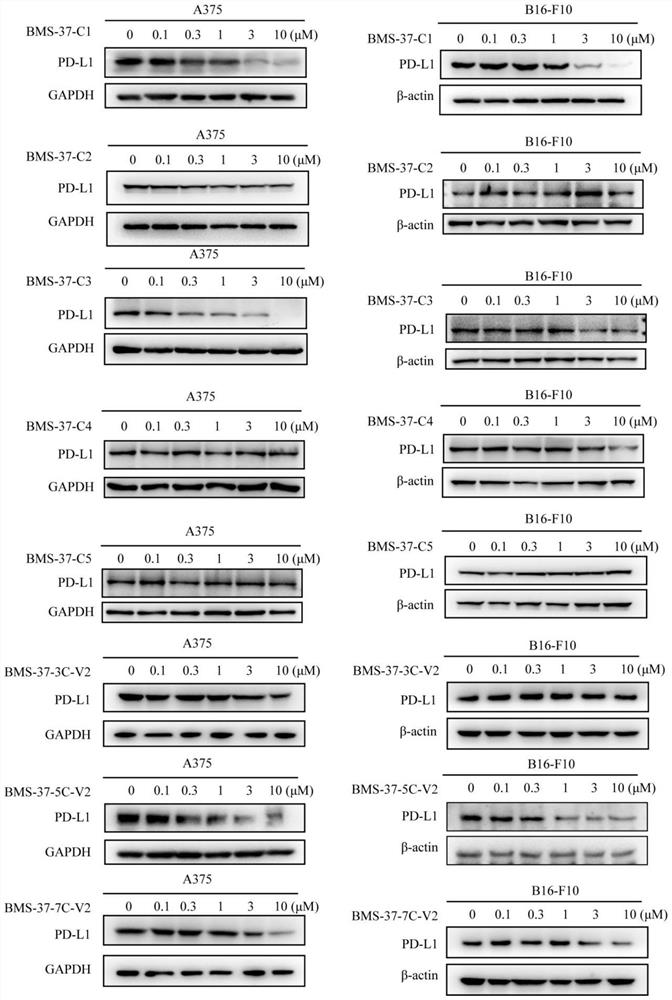
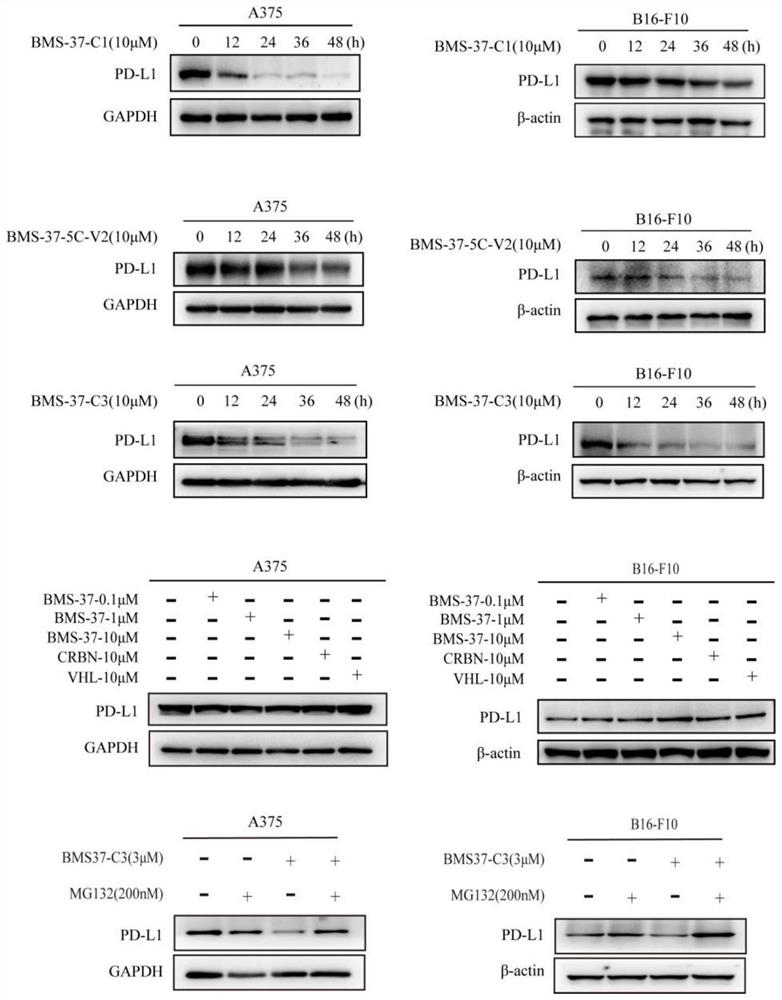
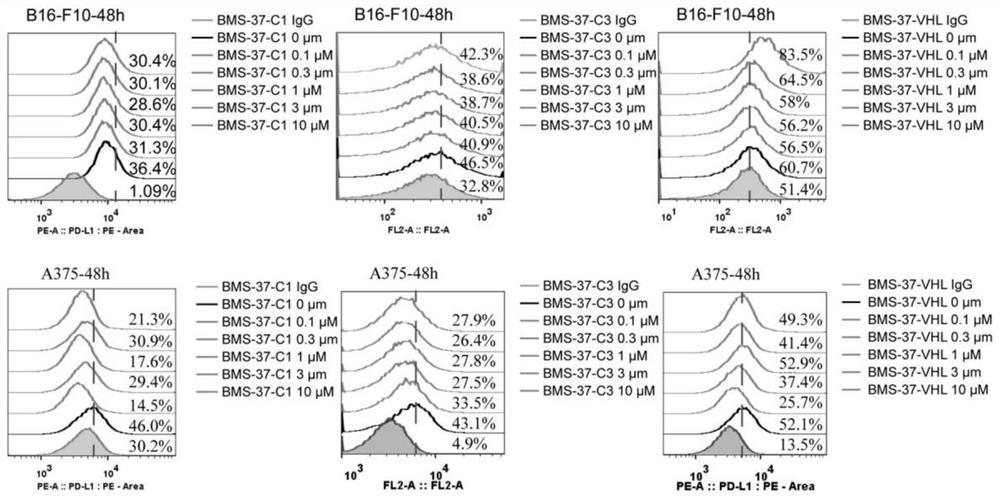
Difunctional molecular compound for inducing degradation of PD-L1 protein as well as preparation and application of difunctional molecular compound
A PD-L1, bifunctional molecule technology, applied in the field of bifunctional molecular compounds, can solve the problems of unsatisfactory efficacy of PD-L1 small molecule inhibitors, and achieve the effect of low onset dose of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Example 1 Synthesis of intermediate compound Th-L2 (m=2)
[0137] In this embodiment, L is m is 2;
[0138] B is CRBN, then the synthetic route is:
[0139]
[0140] 58 mg of compound Th (thalidomide derivative, available for purchase) was placed in an eggplant-shaped flask, 3 mL of DMF was added, 50 mg of azido-2PEG-amine and 47 μL of DIPEA were added in sequence under stirring, and the reaction was carried out at 90 °C for 3-4 hours. Extracted with 30 mL of water and 30 mL of ethyl acetate, the organic layer was dried with anhydrous sodium sulfate, and concentrated to obtain the crude product, which was purified by silica gel column chromatography, eluting with a gradient of petroleum ether-ethyl acetate 1:2 to 1:4 to obtain a yellow oil The compound was 33.2 mg, and the yield was 41%.
[0141] Th-L2, 1 H NMR (400MHz, CDCl 3 )δ9.06(br s,1H),7.49(t,J=7.8Hz,1H),7.09(dd,J=
[0142] 7.2, 2.0Hz, 1H), 6.93 (d, J=8.4Hz, 1H), 6.50 (t, J=5.6Hz, 1H), 4.96–4.92 (m, 1H)...
Embodiment 2
[0143] Example 2 Synthesis of Intermediate Compound M-L1 (m=1)
[0144] In this embodiment, B is MDM2;
[0145] In this embodiment, L is m is 1;
[0146]
[0147]320 mg of compound M was placed in an eggplant-shaped flask, 3 mL of DCM was added, 65 mg of azido-1PEG-amine, 132 μL of DIPEA, 74 mg of EDCI, and 92 mg of HOBt were successively added under ice bath, and the reaction was performed for 3-4 hours, and 30 mL of water and 30 mL of DCM were added for extraction. The organic layer was dried over anhydrous sodium sulfate and concentrated to obtain a crude product, which was purified by silica gel column chromatography to obtain 237.5 mg of a white solid, yield: 63.2%.
[0148] M-L1, 1 H NMR (600MHz, DMSO-d 6 )δ7.96(t,J=5.7Hz,1H),7.56–7.51(m,1H),7.17–
[0149] 7.14(m, 2H), 7.12(d, J=8.3Hz, 2H), 7.07–7.02(m, 2H), 6.98(d, J=8.0Hz, 2H), 6.62(d, J=7.3Hz, 2H) ), 5.66(d, J=9.7Hz, 1H), 5.58(d, J=9.7Hz, 1H), 4.72(hept, J=6.0Hz, 1H), 3.83(s, 4H), 3.76–3.67(m ,2H),3.65–3.55...
Embodiment 3
[0150] The synthesis of embodiment 3 intermediate compound V-2L
[0151] In the present embodiment, B is VHL;
[0152] In this embodiment, L is m is 1;
[0153]
[0154] 215 mg of compound V was placed in an eggplant-shaped flask, 3 mL of DCM was added, 57.5 mg of 3-azidopropionic acid, 132 μL of DIPEA, 74 mg of EDCI, 92 mg of HOBt were sequentially added under ice bath, and the reaction was carried out for 3-4 hours, and 30 mL of water and 30 mL of DCM were added for extraction. , the organic layer was dried over anhydrous sodium sulfate, and concentrated to obtain a crude product, which was purified by silica gel column chromatography to obtain 279 mg of a white solid, yield: 53%.
[0155] V-2L, 1 H NMR (600MHz, DMSO-d 6 )δ8.98(s, 1H), 8.57(t, J=6.0Hz, 1H), 8.12(d, J=9.4
[0156] Hz, 1H), 7.47–7.36 (m, 4H), 5.13 (d, J=3.6Hz, 1H), 4.58 (d, J=9.4Hz, 1H), 4.47–4.40 (m, 2H), 4.39–4.33 (m, 1H), 4.21 (dd, J=15.8, 5.5Hz, 1H), 3.69 (dd, J=10.5, 4.2Hz, 1H), 3.62 (dt, J=10.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com