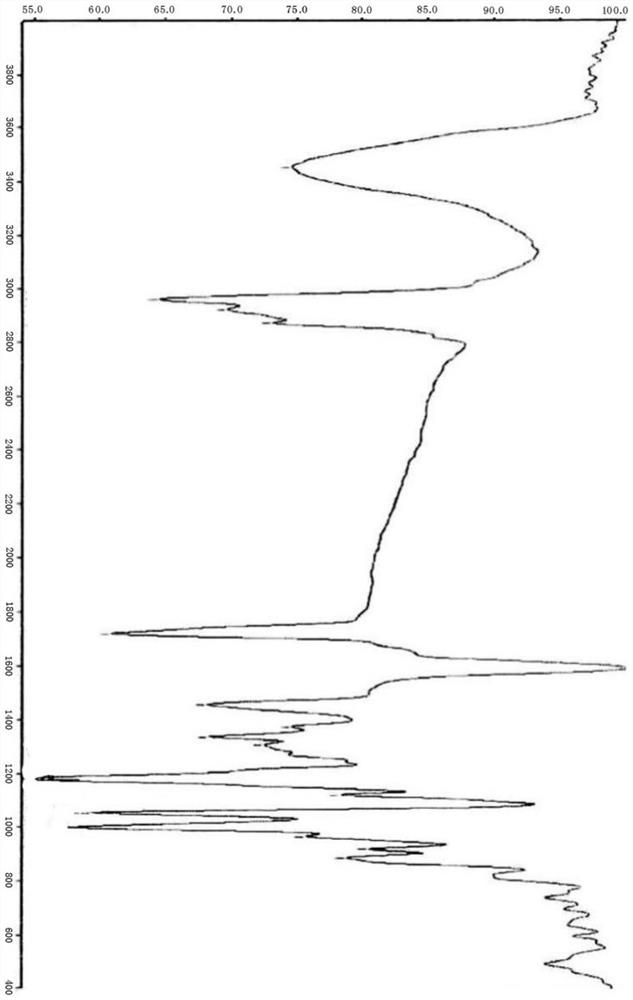
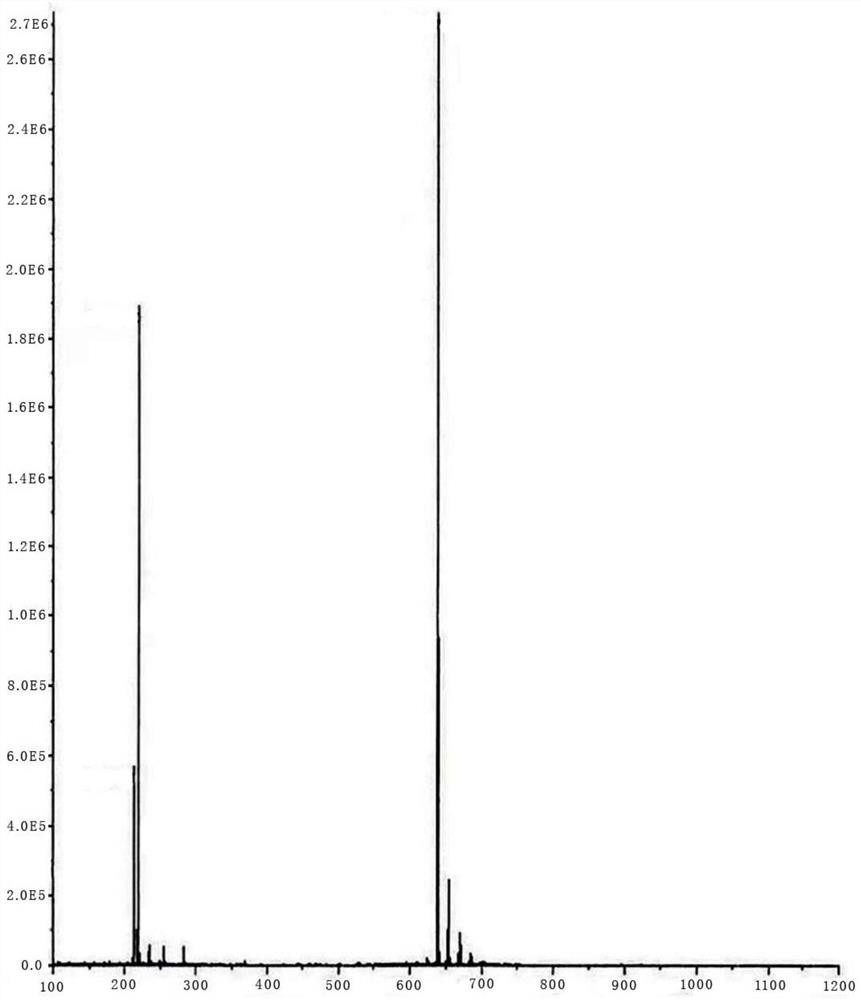
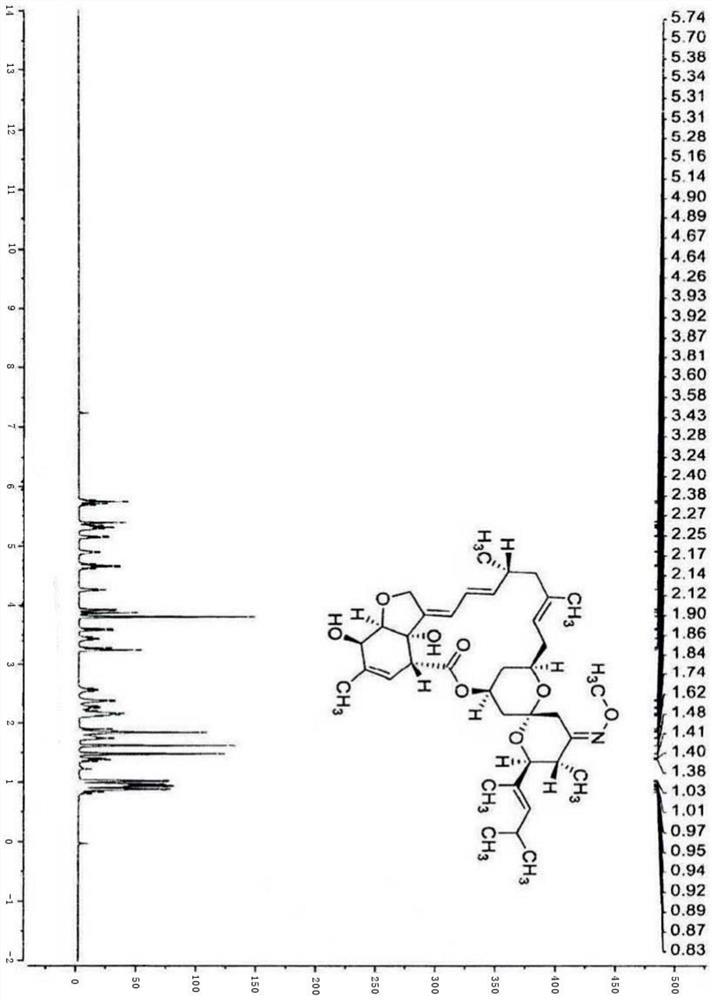
Moxidectin intermediate and preparation method thereof, and preparation method of moxidectin
A technology for moxidectin and intermediates, applied in the field of medicinal chemistry, can solve the problems of expensive protective reagents, low intermediate yields, and unsuitability for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The present embodiment provides a kind of preparation method of moxidectin, comprises the following steps:
[0066] Nucleophilic substitution reaction (protection on hydroxyl group): Synthesis of 5-oxo(β-phenylacryloyl)nemoctine (moxidectin intermediate) from nemoctine
[0067] At room temperature (20-25°C), in a 250mL three-necked flask equipped with a stirrer, add 25g (41.0mmol) nimoctine, 3.3g DMAP (4-dimethylaminopyridine) and 100mL dichloromethane, After stirring and dissolving completely, add triethylamine (17.5mL) and 6.83g β-phenylacryloyl chloride (41.0mmol) successively, stir at room temperature (20-25°C) for 2h until the reaction is complete, then transfer the reaction solution to a 250mL container In a 500mL beaker of sodium bicarbonate solution (5wt%), after stirring for 10min to uniformity, then stand for 20min to separate layers, move the lower organic phase to a 250mL Erlenmeyer flask, add 50g of anhydrous magnesium sulfate for drying, and place overnigh...
Embodiment 2
[0087] The present embodiment provides a kind of preparation method of moxidectin, comprises the following steps:
[0088] Nucleophilic substitution reaction (protection on hydroxyl group): Synthesis of 5-oxo(β-phenylacryloyl)nemoctine (moxidectin intermediate) from nemoctine
[0089] At room temperature (20-25°C), in a 250mL three-necked flask equipped with a stirrer, add 25g (41mmol) nimoctine, 3.5g DMAP (4-dimethylaminopyridine) and 100mL dichloromethane, stir After completely dissolving, add triethylamine (25mL) and 6.83g β-phenylacryloyl chloride (41mmol) successively, stir at room temperature (20-25°C) for 1.75h until the reaction is complete, then transfer the reaction liquid to 250mL bicarbonate In a 500mL beaker of sodium solution (5wt%), stir for 10min until uniform, then let stand for 20min to separate layers, move the lower organic phase to a 250mL Erlenmeyer flask, add 50g of anhydrous magnesium sulfate to dry, and place overnight. The dried liquid was filtered a...
Embodiment 3
[0097] The present embodiment provides a kind of preparation method of moxidectin, comprises the following steps:
[0098] Nucleophilic substitution reaction (protection on hydroxyl group): Synthesis of 5-oxo(β-phenylacryloyl)nemoctine (moxidectin intermediate) from nemoctine
[0099] At room temperature (25-30°C), in a 250mL three-neck flask equipped with a stirrer, add 25g (41mmol) nimoctine, 3.0g DMAP (4-dimethylaminopyridine) and 100mL dichloromethane, stir After complete dissolution, add triethylamine (34mL) and 6.83g β-phenylacryloyl chloride (41mmol) in sequence, stir at room temperature (25-30°C) for 1.5h until the reaction is complete, then transfer the reaction solution to 250mL bicarbonate In a 500mL beaker of sodium solution (5wt%), stir for 10min until uniform, then let stand for 20min to separate layers, move the lower organic phase to a 250mL Erlenmeyer flask, add 50g of anhydrous magnesium sulfate to dry, and place overnight. The dried liquid was filtered and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com