sgRNA and method for constructing gm-csf(-) cells using the same
A GM-CSF, cell technology, applied in the field of sgRNA and the construction of GM-CSF cells by using it, can solve the problem of lack of CRS treatment methods, and achieve the effect of preventing the occurrence of CRS and improving the curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: In vitro transcription (IVT) of CD19 FMC63 CAR mRNA
[0042] 1. The pDA-FMC63 CAR plasmid (NEB, Cat: R3133L) was linearized by SpeI digestion.
[0043] 2. The linearized vector was purified with a PCR cleanup kit (Qiagen) and eluted with RNase-free water.
[0044] 3. DNA concentration was measured by Nanodrop and checked by running an agarose DNA gel.
[0045] 4. In vitro transcription (IVT) was performed according to the manufacturer's standard operating procedure (Thermofisher, Cat. No. AMB13455). Briefly, 1 μg of template DNA, NTP / ARCA buffer, T7 buffer, GTP, T7 enzyme, and RNase-free H 2 O was added to a 0.2 mL PCR tube in a volume of 20 μl and incubated for 4 h at 37 °C.
[0046] After 5.4 hours, add 2 microliters of DNase I per reaction and react at 37°C for 15 minutes.
[0047] 6. The PolyA tailing procedure was then performed according to the manufacturer's standard operating procedure.
[0048] 7. IVT mRNA was purified using RNasy kit (Qiagen) (...
Embodiment 2
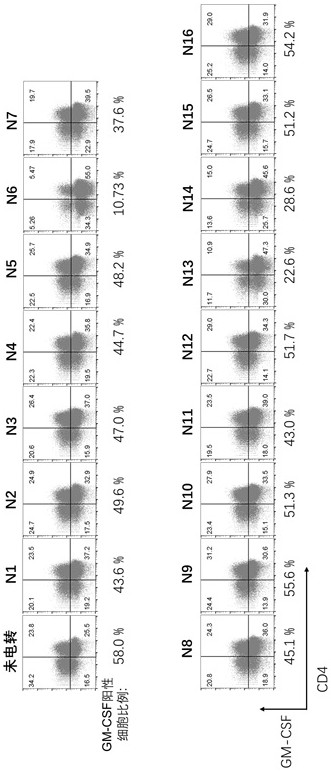
[0050] Example 2: Electroporation of Cas9 / GM-CSF gRNA to generate GM-CSF KO T cells
[0051] 1. On day 1, T cells were isolated from PBMCs and activated by anti-CD3 / CD28 magnetic beads (Thermofisher, catalog number: 402031 ) at a 1:3 ratio of T cells to magnetic beads.
[0052] 2. On day 4, the magnetic beads were removed from the T cells and washed twice with OPTI-MEM, and the cells were resuspended with OPTI-MEM at a concentration of 6e7 / ml.
[0053] 3. RNP complexes were prepared by mixing sgRNA and Cas9 protein (Thermofisher, Cat: A36499) at the desired ratio (GM-CSF gRNA 7.5 μg, Cas9 protein 15 μg / 100 μl electroporation) and incubated at room temperature for 10 min.
[0054] 4. Mix 100 microliters of cells with the RNP complex, then transfer the cell and RNP mixture into a 0.2 cm electroporation cup.
[0055] 5. Set parameters on the BTX ECM 830 machine: 360 voltage, 1 ms for electroporation, then transfer cells to pre-warmed medium and incubate at 37°C.
[0056] 6. Che...
Embodiment 3
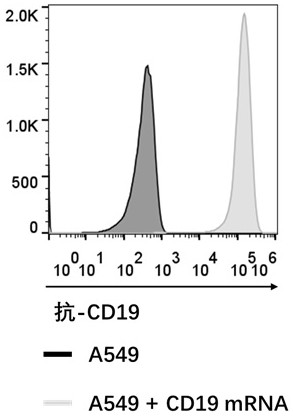
[0058] Example 3: Electroporation of mRNA into A549-GFP tumor cells and T cells
[0059] 1. A549-GFP tumor cells and T cells were collected and washed 3 times with Opti-MEM medium. Among them, A549-GFP cells are in A549 cells (ATCC, CCL-185 TM , https: / / www.atcc.org / products / ccl-185), the specific method is: Infect A549 cells with GFP-expressing lentivirus, and then use flow cytometry to sort GFP-positive cells , and then obtain the A549-GFP cell line.
[0060] 2. Resuspend the cell pellet with Opti-MEM medium and adjust the cell concentration to 1×10e7 / ml.
[0061] 3. Add 5 micrograms of CD19 mRNA, 10 micrograms of anti-CD19 FMC63 CAR mRNA, etc. into 1.5 ml EP tubes, respectively, and then add 100 microliters of A549 cells or T cells, and mix well.
[0062] 4. Set parameters on BTX ECM 830 machine:
[0063] a) For T cells: 500 voltage, 0.7 ms;
[0064] b) For A549-GFP tumor cells: 300 voltage, 0.5ms;
[0065] 5. Add 100 microliters of cells mixed with RNA to the BTX ele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com