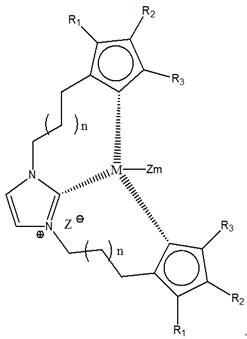
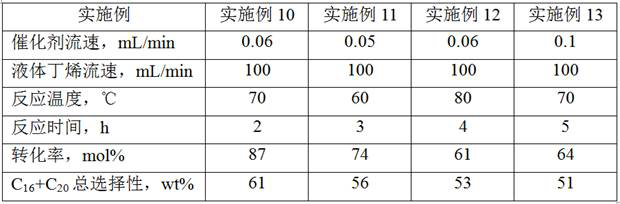
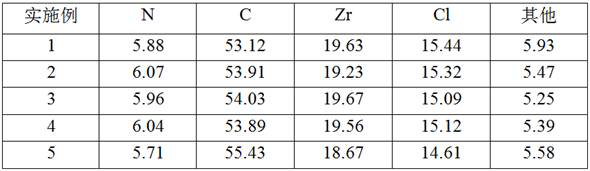
Imidazole bridged metallocene, catalyst and preparation and application thereof
A metallocene catalyst, metallocene technology, applied in the direction of metallocene, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc., can solve the problem that high-value solvent oil cannot be produced, cannot be obtained, reduce Special solvent oil value and other issues, to achieve the effect of reducing catalyst cost, improving selectivity, and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Add 66g cyclopentadiene (66g / mol) to tetrahydrofuran, cool to -40°C, then dropwise add 64g butyllithium (64g / mol), stir for 6h, then add 127g 1,4-dichloro Butane (127 g / mol). Stirring was continued for 10 h.
[0054] (2) 34g of imidazole (68g / mol), 156g of chlorobutylcyclopentadiene (156g / mol) and 106g of sodium carbonate (106g / mol) were added to 680g of acetonitrile, heated and stirred at a temperature of 70°C and reacted for 24h, The solid was removed by filtration, and the liquid was separated by rotary distillation to obtain N,N-dicyclopentadienylbutylimidazole.
[0055] (3) Dissolve 86 g of N,N-dicyclopentadienylbutylimidazole (345 g / mol) in 3450 g of tetrahydrofuran, and cool to -40°C. After stirring, 48 g of n-butyllithium (64 g / mol) was added dropwise, and the reaction was stirred for 5 h. Then, 58 g of zirconium tetrachloride (233 g / mol) was added thereto, followed by stirring for 24 hours.
[0056] (4) Drain the tetrahydrofuran in the solution, add 603...
Embodiment 2
[0062] (1) Add 66g cyclopentadiene (66g / mol) to tetrahydrofuran, cool to -40°C, then dropwise add 76.8g butyllithium (64g / mol), stir for 3h, then add 127g 1,4-dichloro butane (127 g / mol). Stirring was continued for 10 h. Distillation separation to obtain chlorobutylcyclopentadiene.
[0063] (2) 34g of imidazole (68g / mol), 187g of chlorobutylcyclopentadiene (156g / mol) and 159g of sodium carbonate (106g / mol) were added to 1700g of acetonitrile, heated and stirred at a temperature of 50°C for 24h, The solid was removed by filtration, and the liquid was separated by rotary distillation to obtain N,N-dicyclopentadienylbutylimidazole.
[0064] (3) 86 g of N,N-dicyclopentadienylbutylimidazole (309 g / mol) was dissolved in 4299 g of tetrahydrofuran, and cooled to -40°C. After stirring, 56 g of n-butyllithium (64 g / mol) was added dropwise, and the reaction was stirred for 5 h. Then, 70 g of zirconium tetrachloride (233 g / mol) was added thereto, followed by stirring for 24 hours.
...
Embodiment 3
[0071] (1) Add 66g of cyclopentadiene (66g / mol) to tetrahydrofuran, cool to -40°C, then dropwise add 51.2g of butyllithium (64g / mol), stir for 3h, then add 101.6g of 1,4-di Chlorobutane (127 g / mol). Stirring was continued for 10 h.
[0072] (2) 34g of imidazole (68g / mol), 140g of chlorobutylcyclopentadiene (156g / mol) and 53g of sodium carbonate (106g / mol) were added to 340g of acetonitrile, heated and stirred at a temperature of 80°C for 24h, The solid was removed by filtration, and the liquid was separated by rotary distillation to obtain N,N-dicyclopentadienylbutylimidazole.
[0073] (3) Dissolve 86 g of N,N-dicyclopentadienylbutylimidazole (309 g / mol) in 860 g of tetrahydrofuran, and cool to -40°C. After stirring, 44.8 g of n-butyllithium (64 g / mol) was added dropwise, and the reaction was stirred for 5 h. Then, 46.6 g of zirconium tetrachloride (233 g / mol) was added thereto, followed by stirring for 24 hours.
[0074] (4) Drain the tetrahydrofuran in the solution, add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com