Positive electrode active material and non-aqueous electrolyte secondary battery
A positive electrode active material and secondary battery technology, which is applied in the direction of non-aqueous electrolyte batteries, active material electrodes, secondary batteries, etc., can solve the problems that cannot fully meet the cycle performance and rate performance, and cannot fully meet the energy density. Reduced size, excellent cycle performance and rate performance, and high energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0277] Hereinafter, the present invention will be specifically described with reference to representative examples and comparative examples of the present invention, but the present invention is not limited to these examples.
[0278] "Examples and Comparative Examples Relating to Present Invention I"
Embodiment I-1
[0280] A 0.1 mol / L nickel sulfate aqueous solution and a 0.1 mol / L manganese sulfate aqueous solution were prepared. The above-mentioned nickel sulfate aqueous solution and the above-mentioned manganese sulfate aqueous solution were mixed so that the molar ratio of nickel and manganese was Ni:Mn=0.35:0.65 to obtain a mixed solution. Prepare a 1 mol / L sodium carbonate aqueous solution. 8 L of water was added to the closed-type reaction tank, nitrogen gas was introduced, and the temperature was maintained at 40°C. The above-mentioned mixed solution and the above-mentioned sodium carbonate aqueous solution were continuously dropped into the above-mentioned reaction tank at a rate of 5 mL / min while stirring. At the same time, the above-mentioned sodium carbonate aqueous solution was added dropwise so that pH=8.00 (±0.01). During the reaction, only the filtrate was discharged out of the reaction system by the concentrator, and the solid content was left in the reaction tank, and ...
Embodiment I-2
[0293] In Example I-1, a mixed solution of nickel sulfate aqueous solution, cobalt sulfate aqueous solution and manganese sulfate aqueous solution was added, so that the molar ratio of nickel, cobalt and manganese was Ni:Co:Mn=0.35:0.05:0.60. Example I-1 also obtained the powder of the coprecipitated precursor.
[0294] The resulting co-precipitated precursor is (Ni 0.35 Co 0.05 Mn 0.60 )CO 3 (carbonate precursor compound). The lithium carbonate powder was weighed so that the ratio (molar ratio) Li / (Ni+Co+Mn) of lithium to the coprecipitated precursor was 1.25, and the coprecipitated precursor was thoroughly mixed. The mixture was calcined in an oxidizing atmosphere at 850° C. for 5 hours using an electric furnace to obtain a positive electrode active material.
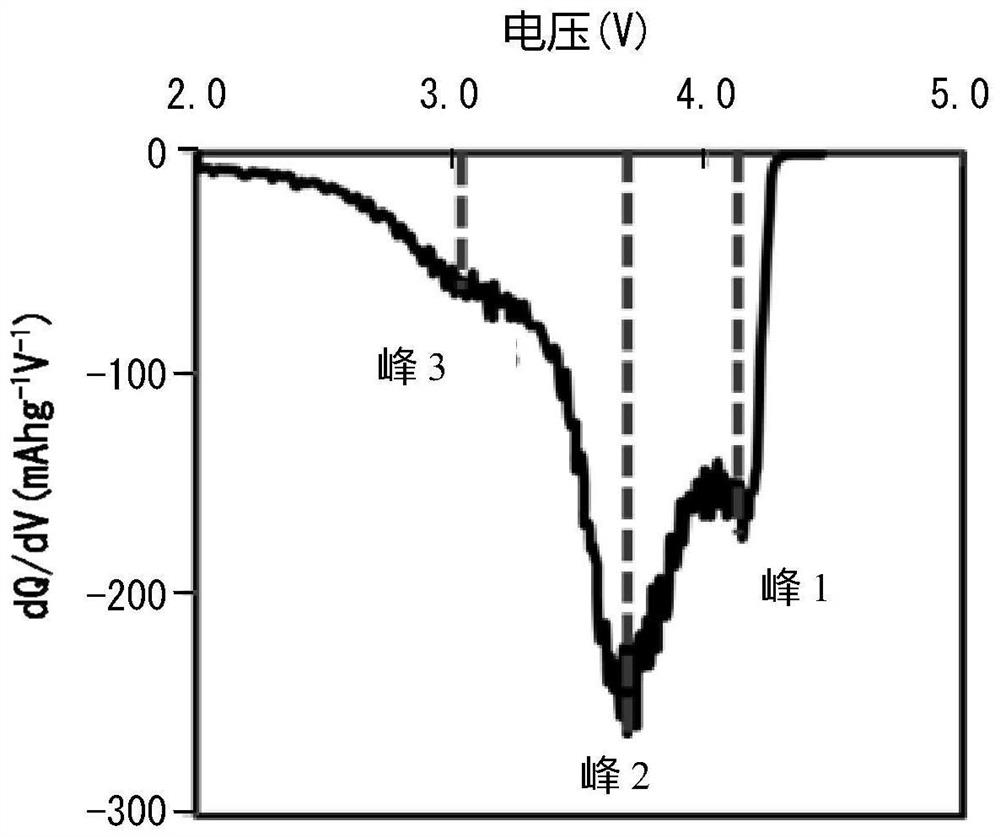
[0295] Using the obtained positive electrode active material, a button battery was produced in the same manner as in Example I-1, and a graph was drawn with the voltage V on the horizontal axis and the dQ / dV valu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com