Polymerizable benzophenone photoinitiator and preparation method thereof
A technology of benzophenones and photoinitiators, which is applied in the field of polymerizable benzophenone photoinitiators and its preparation, can solve the problems of unfavorable application and popularization of polymerizable photoinitiators, long routes, and high costs. Achieve the effect of reducing surface migration and toxicity, simple synthesis method, and no post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The present invention also provides a preparation method of a polymerizable benzophenone photoinitiator, comprising the following steps:
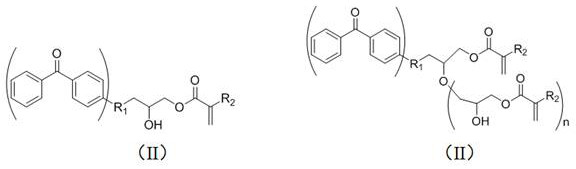
[0046] The target products (I) and (II) are obtained by heating and stirring the benzophenone derivative A and the epoxy acrylate compound B in the presence of a catalyst and a polymerization inhibitor. The reaction formula is as follows:
[0047]
[0048] A is a benzophenone derivative, and B is an epoxy acrylate compound;
[0049] (I) is a mono-epoxy reaction product, (II) is a poly-epoxy reaction product;
[0050] Wherein, R1 represents oxygen, ethoxy of O(CH2)2O, and propoxy of O(CH2)3O.
[0051] The substitution of R1 in the present embodiment on benzophenone is mono-substituted or polysubstituted, and the position of R1 includes ortho-position, para-position and meta-position relative to the carbonyl position of benzophenone;
[0052] Described R2 represents hydrogen or methyl;
[0053] The n is greater than or equal to 1...
Embodiment 1
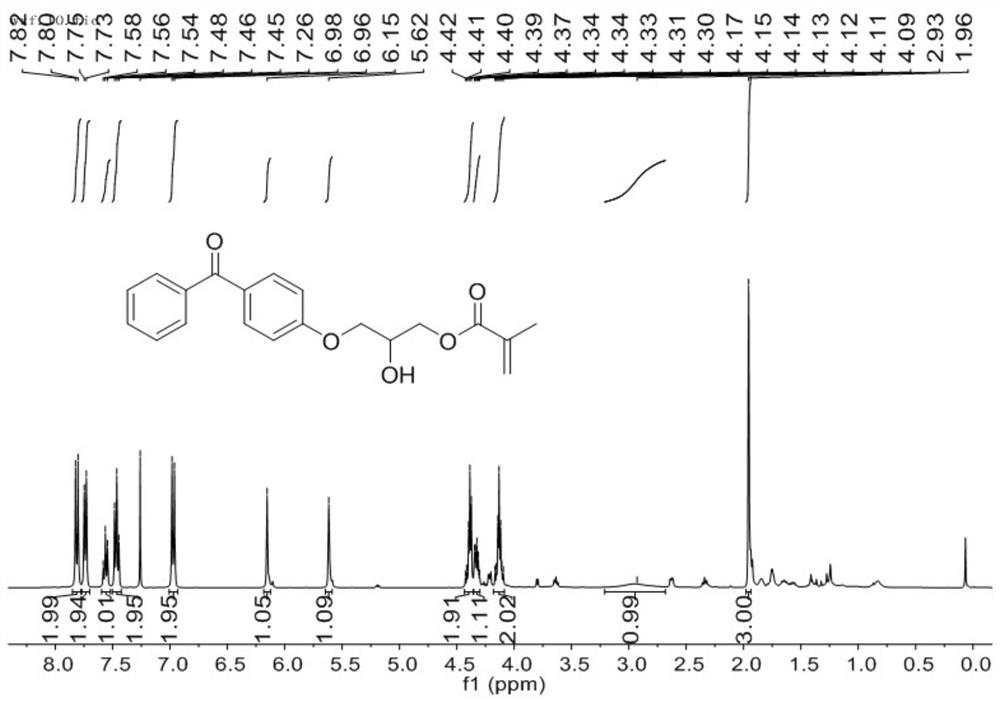
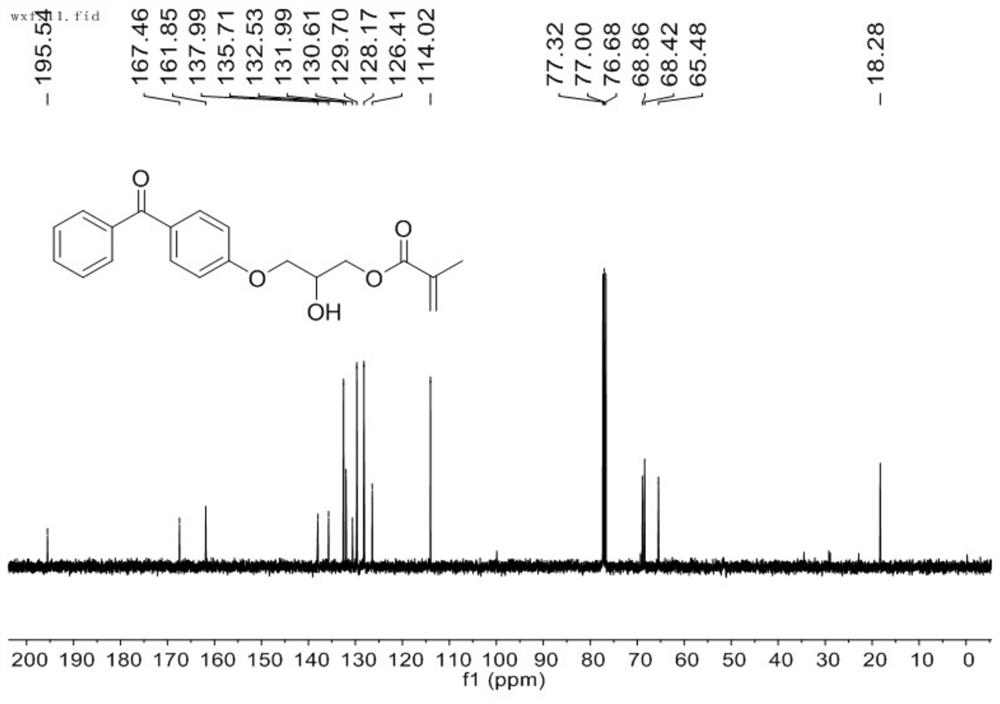
[0063] Take 100 mL, 0.1 mol (19.82 g) of 4-hydroxybenzophenone, 0.2 mol (28.43 g) of glycidyl methacrylate, 24 mg of p-hydroxyanisole, and 480 mg of triphenylphosphine, stir at 100 ° C for 6 h, pass The reaction was monitored by thin layer chromatography until the reaction of the starting material was complete. The resulting product was a yellow oily liquid comprising a mixture of mono- and poly-epoxy reaction products. The reaction product is processed by column chromatography to obtain a mono-epoxy reaction product. figure 1 and figure 2 They are the nuclear magnetic H spectrum and the nuclear magnetic C spectrum of the mono-epoxy reaction product prepared in this example. figure 1The chemical shifts of the peaks in the hydrogen spectrum 1H NMR (400 MHz, CDCl3) are δ7.81 (d, J = 8.8 Hz, 2H), 7.74 (d, J = 7.1 Hz, 2H), 7.56 (t, J =7.4 Hz, 1H), 7.46 (t, J = 7.5 Hz, 2H), 6.97 (d, J = 8.8 Hz, 2H), 6.15 (s, 1H), 5.62 (s, 1H), 4.43 – 4.36 (m, 2H) ), 4.32 (dt, J = 9.7, 3.9 Hz, ...
Embodiment 2
[0065] Take 100mL, 4-hydroxybenzophenone 0.1mol (19.82g), glycidyl methacrylate 0.2mol (28.43g), p-hydroxyanisole 24mg, benzyltrimethylammonium chloride 480mg, at 100 ℃ Stir for 6 h and monitor the reaction by thin layer chromatography until the reaction of the starting material is complete. The product was obtained as a yellow oily liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com