Low-reactivity polyaspartic acid ester resin and preparation method thereof, and coating and preparation method thereof
An aspartate, reactive technology, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems of low reactivity and product performance, difficult to balance and other problems, achieve excellent application effect, wide application field, preparation Simple and controllable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
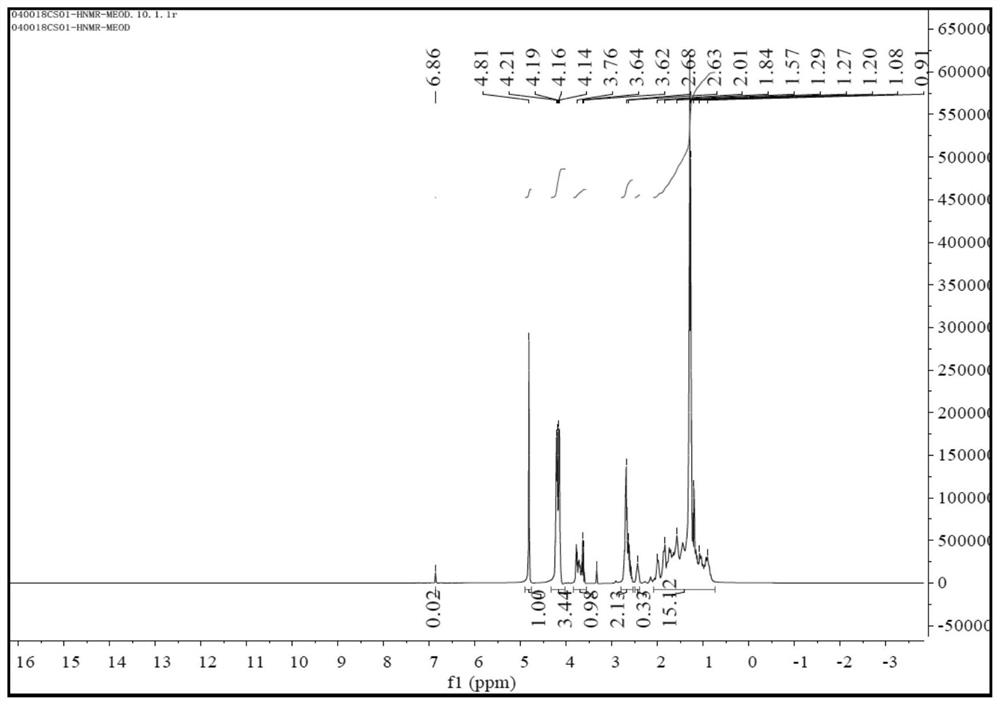
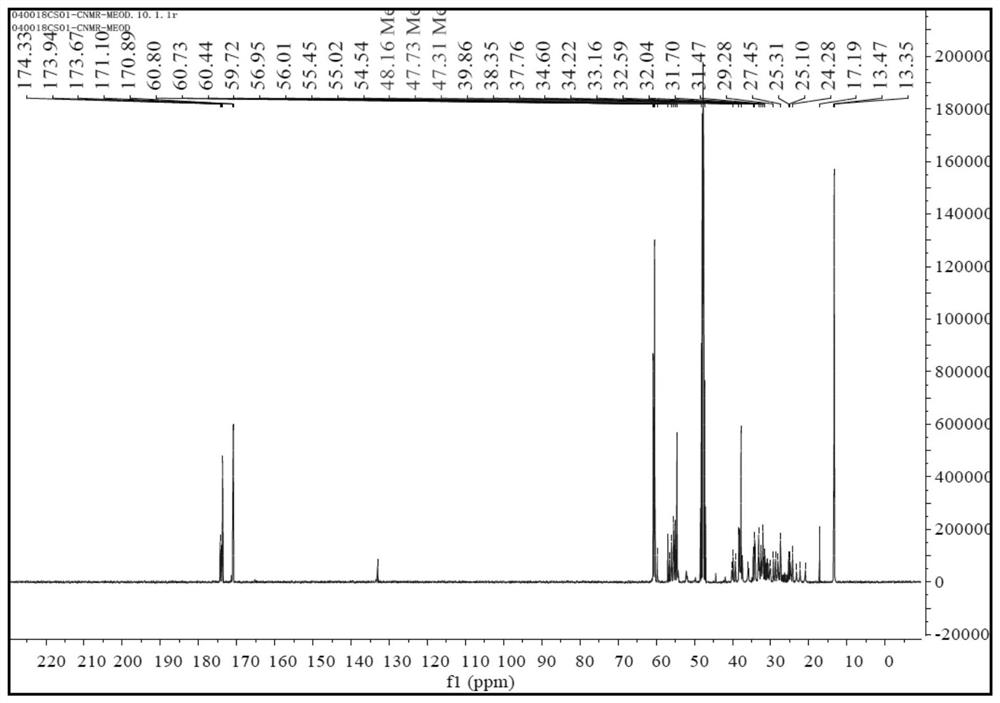
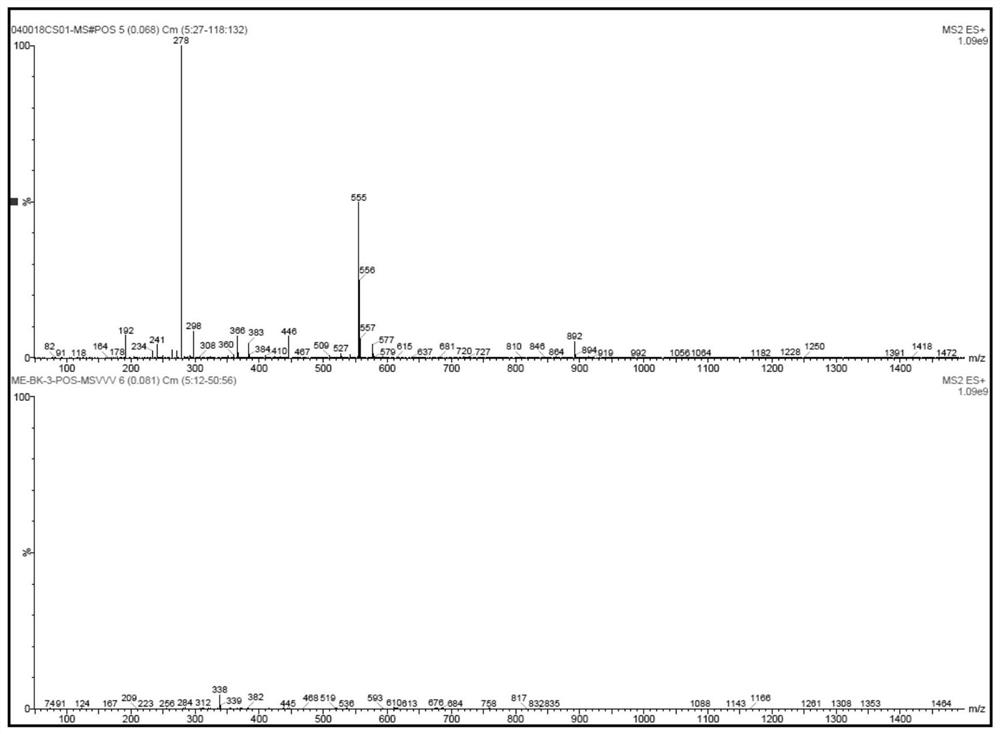
[0087] Add 210g (1mol) H-7011 (2,2'-diaminodicyclohexylmethane content is 35%; 2,4'-diaminodicyclohexylmethane content is 65%; molar mass is 210) into the reaction vessel , 344g (2mol) of diethyl maleate was added dropwise to H-7011, and the temperature of the dropwise addition was controlled not to be higher than 60°C. Under the conditions of 130°C and vacuum degree higher than -0.098MPa, it was purified by negative pressure distillation in a thin film evaporator, then cooled to 50°C and filtered to obtain 548.5g of polyaspartate resin with a yield of 99% and a molecular weight of 554. , the active hydrogen equivalent is 277;
[0088] The 1H-NMR spectrum, 13C-NMR spectrum, MS(ES+) spectrum and MS(ES-) spectrum of the prepared polyaspartate resin are shown in Figure 1 to Figure 4 ;
[0089] The obtained polyaspartate resin is a mixture of substances represented by structural formula (3) and structural formula (4):
[0090]
[0091] The content of structural formula (3) ...
Embodiment 2
[0093] The difference with Example 1 is that diethyl maleate was replaced with equimolar di-n-butyl maleate 456g (2mol), and the rectification and purification temperature was 140° C. to obtain polyaspartate resin 659g, The yield is 99%, the molecular weight is 666, and the active hydrogen equivalent is 333;
[0094] The obtained polyaspartate resin is a mixture of substances represented by structural formula (5) and structural formula (6):
[0095]
[0096] Wherein, the content of structural formula (5) is 35%, and the content of structural formula (6) is 65%.
Embodiment 3
[0098] The difference with Example 1 is that the consumption of diethyl maleate is 516g (3mol), that is, the mol ratio of H-7011 and diethyl maleate is 1:3, to obtain polyaspartate resin 549.3 g, the yield is 75.7%, and its molecular weight, active hydrogen equivalent and molecular structure are the same as those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com