Kit for detecting respiratory syncytial virus and application thereof
A syncytial virus and kit technology, which is applied in the directions of microorganism-based methods, microbial determination/inspection, biochemical equipment and methods, etc., can solve the problem that the detection sensitivity of RPA technology has not been effectively improved, and the detection operation of enzyme-linked immunosorbent assay Complex, high detection costs, to achieve good application prospects, short detection time, easy to operate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
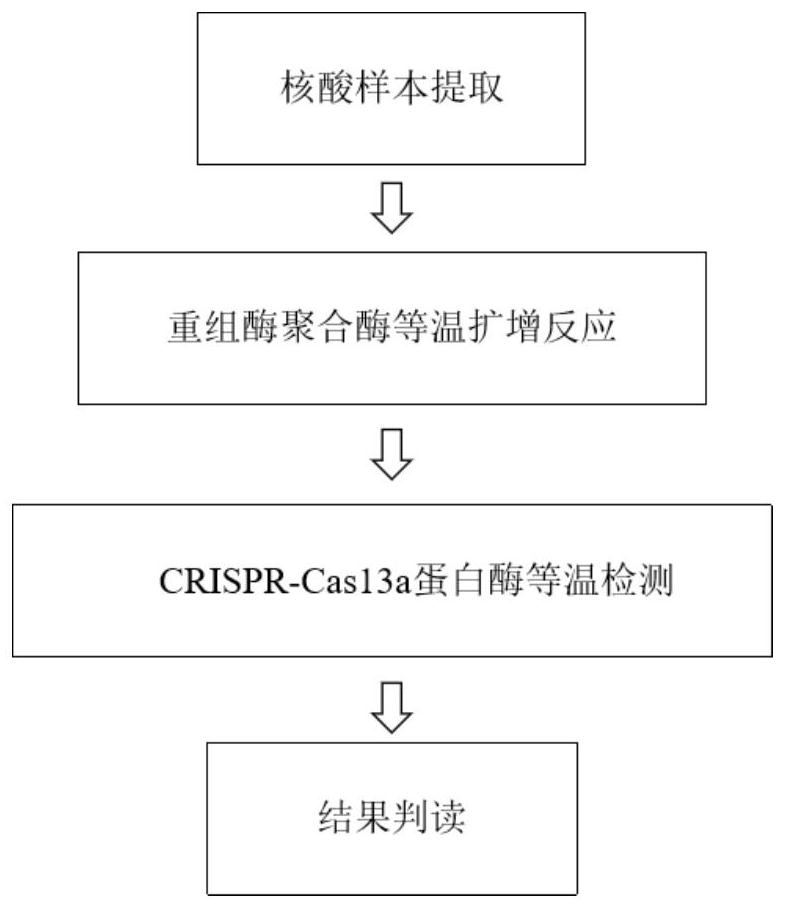
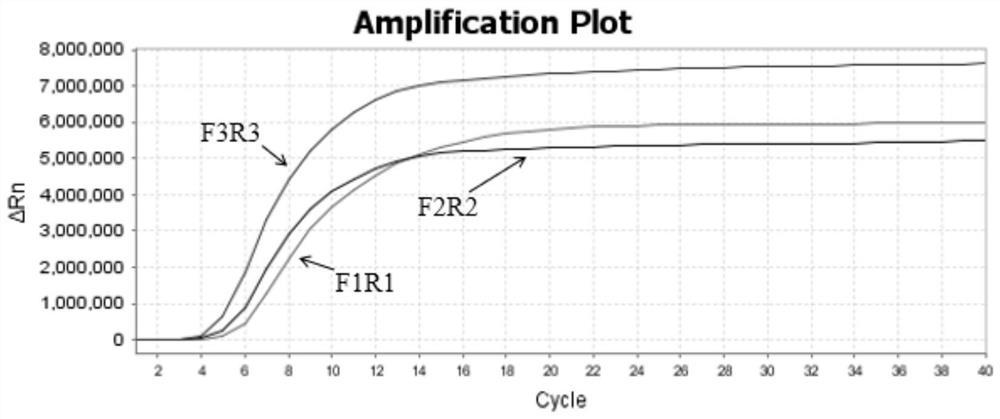
[0047] In this example, the amplification efficiency of the RPA amplification primer pair for respiratory syncytial virus was studied. Under the condition that the contents of each component of the reaction system did not change, the amplification efficiency of three pairs of respiratory syncytial virus RPA primer pairs was studied.
[0048] The amplification reaction system was: 1 μL RSV-F (10 μM), 1 μL RSV-R (10 μM), 0.3 μL 1×syto-9, 20.5 μL RPA Buffer, 1.2 μL Mg(CH3COO)2 (280 mM), template (containing respiratory tract cytovirus detection target sequence T vector) 50ng, make up to 25μL with ultrapure water. The amplification program was set to 42°C, pre-denaturation for 30s; denaturation at 42°C for 15s, annealing and extension at 37°C for 15s, with 40 cycles.
[0049] The sequences of RPA amplification primer pairs for respiratory syncytial virus are shown in Table 1.
[0050] Table 1 Respiratory syncytial virus RPA amplification primer pair sequences
[0051]
[005...
Embodiment 2
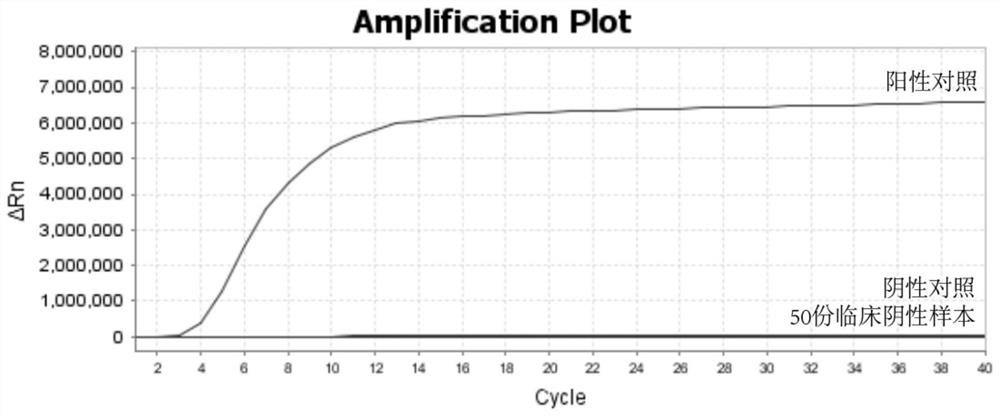
[0054] In this example, 50 clinically negative samples of respiratory syncytial virus were used as specificity evaluation indicators to analyze the specificity of the RPA amplification primer pair for respiratory syncytial virus provided in Example 1. According to the RPA amplification reaction system and amplification procedure described in Example 1, 50 clinically negative samples of respiratory syncytial virus were amplified, and a positive control and a negative control were set at the same time. Among them, the template of the positive control is a T vector containing the target sequence of respiratory syncytial virus detection; the template is not added to the negative control.
[0055] Test results such as image 3 shown, according to image 3 , only the positive control showed an "S"-shaped amplification curve, and the negative control and 50 clinically negative samples did not show amplification. It shows that the RPA amplification primer pair provided by the presen...
Embodiment 3
[0057] This embodiment provides a kit for detecting respiratory syncytial virus, including an RPA reaction system and a CRISPR-Cas13a protease detection system.
[0058] The RPA reaction system is shown in Table 2.
[0059] Table 2 RPA reaction system
[0060]
[0061]
[0062] The CRISPR-Cas13a protease detection system is shown in Table 4.
[0063] Table 3 Base sequences required for CRISPR-Cas13a protease detection
[0064]
[0065] Table 4 CRISPR-Cas13a protease detection system
[0066] Reagent ingredients Amount used (μL) NTP Buffer Mix 10 T7 transcriptase 3 sgRNA 1 report sequence 5 Mg(CH 3 COO) 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com