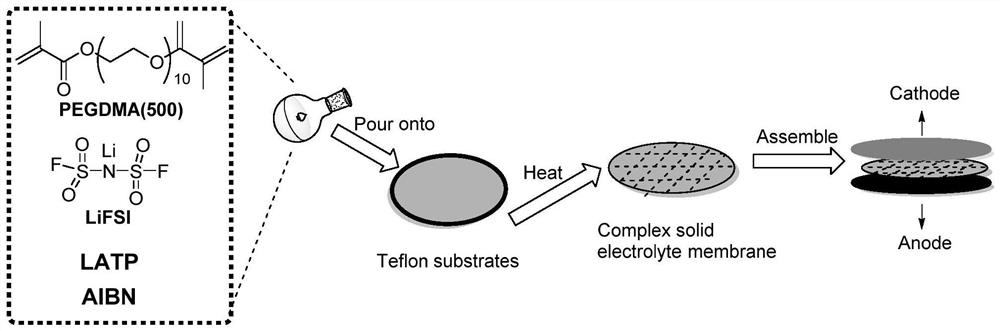
In-situ polymerized polymer solid electrolyte as well as preparation method and application thereof
A solid-state electrolyte and in-situ polymerization technology, applied in circuits, electrical components, secondary batteries, etc., can solve problems such as the gap in large-scale commercial applications, and achieve the effect of improving safety performance and improving ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1, in-situ polymerized PEO cross-linked main chain structure polymer electrolyte preparation (process such as figure 1 Shows):
[0039] (1) The design reaction monomer is: a bifunctional monomer and R 1 for CH 3 , that is, the structure is a bifunctional monomer with methacrylate groups at both ends, the number of repeating units in the middle segment (C-C-O) is n is 13, and the polymer name is polyethylene glycol dimethacrylate (PEGDMA-n13) ; Mix the reaction monomer 9.9g with the additive VC 0.1g, stir for 12h to make it evenly dispersed;
[0040] (2) Lithium salt LiTFSI (0.5 g) was added to the mixed liquid, stirred at 60° C. for 48 hours, and stood for 48 hours.
[0041](3) The initiator AIBN (0.05 g) was added to the mixed solution, stirred for 5 minutes, and then rapidly injected into the cell for in-situ polymerization by applying a vacuum-pumping liquid injection process.
[0042] (4) The battery core is evacuated before the electrolyte is injected, ...
Embodiment 2
[0046] Example 2: The only difference between Example 2 and Example 1 is that n=9 in the bifunctional monomer in the reaction monomer, and the polymer name is polyethylene glycol dimethacrylate (PEGDMA-n9), Preparation of solid electrolyte film, the test ionic conductivity is 9.84*10 -6 S / cm. Studies have shown that too small a degree of polymerization can not complete the purpose of migrating ions, while too large a degree of polymerization will affect its crystallinity, so it cannot meet the requirements of the subsequent in-situ polymerization process. After bulk polymerization, it can not only meet the requirements of in-situ polymerization, but also improve the electrical conductivity. When n=13, the ionic conductivity is the highest.
Embodiment 3
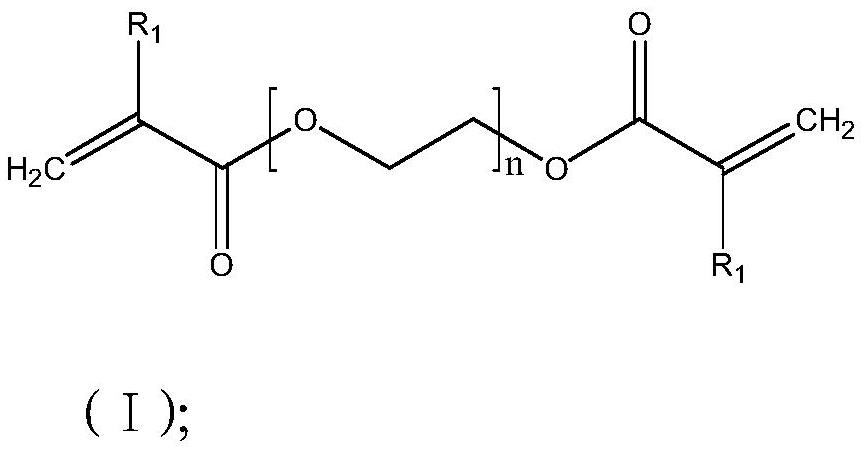
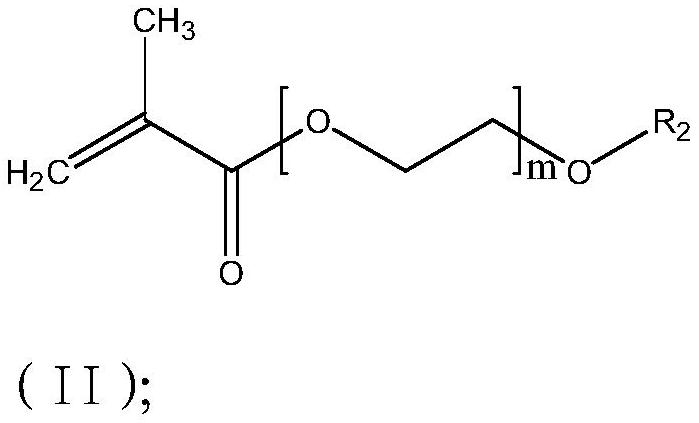
[0047] Example 3: The only difference between Example 3 and Example 1 is that the reaction monomer is a mixture of a bifunctional monomer and a monofunctional monomer, wherein the bifunctional monomer is the compound of formula (I), and R 1 for CH 3 , n=13, the polymer name is polyethylene glycol dimethacrylate (PEGDMA-n13); the monofunctional monomer is the compound of formula (II), and R 2 is H, m=9, and the polymer name is polyethylene glycol methacrylate (PEGMA-n9); the mixing ratio of difunctional monomer and monofunctional monomer is 80:19. Preparation of solid electrolyte film, the test ionic conductivity is 1.25*10 -5 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com