Preparation method and application of metformin carbon dots
A metformin carbon dot and metformin technology, which is applied to medical preparations containing active ingredients, pharmaceutical formulations, carbon active ingredients, etc., can solve the problems of low functional neuron efficiency, limiting the wide application of neural stem cells, and decreasing neural stem cell survival rate. , to achieve the effect of promoting efficiency and maturity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] In order to make the objectives, technical solutions and advantages of the present invention clearer, the present invention will be further described in detail below with reference to the accompanying drawings and embodiments. The embodiments listed in the present invention are only used to illustrate the present invention, but not to limit the scope of the present invention. Any obvious modifications or changes made to the present invention do not depart from the spirit and scope of the present invention.
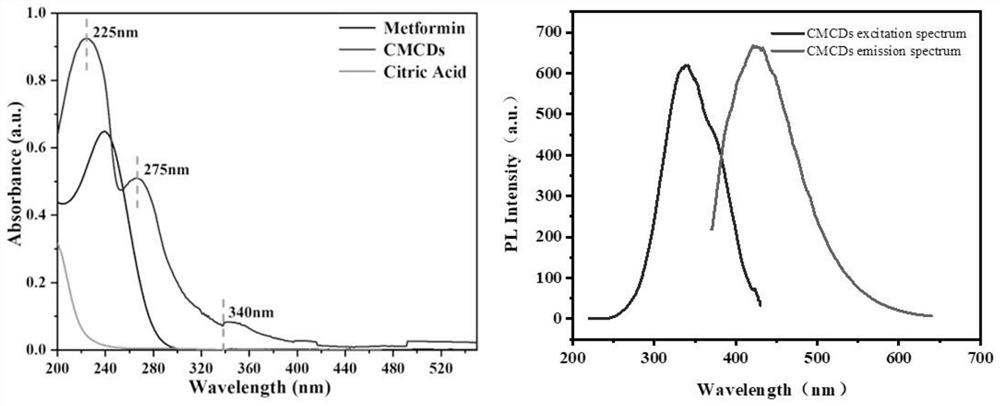
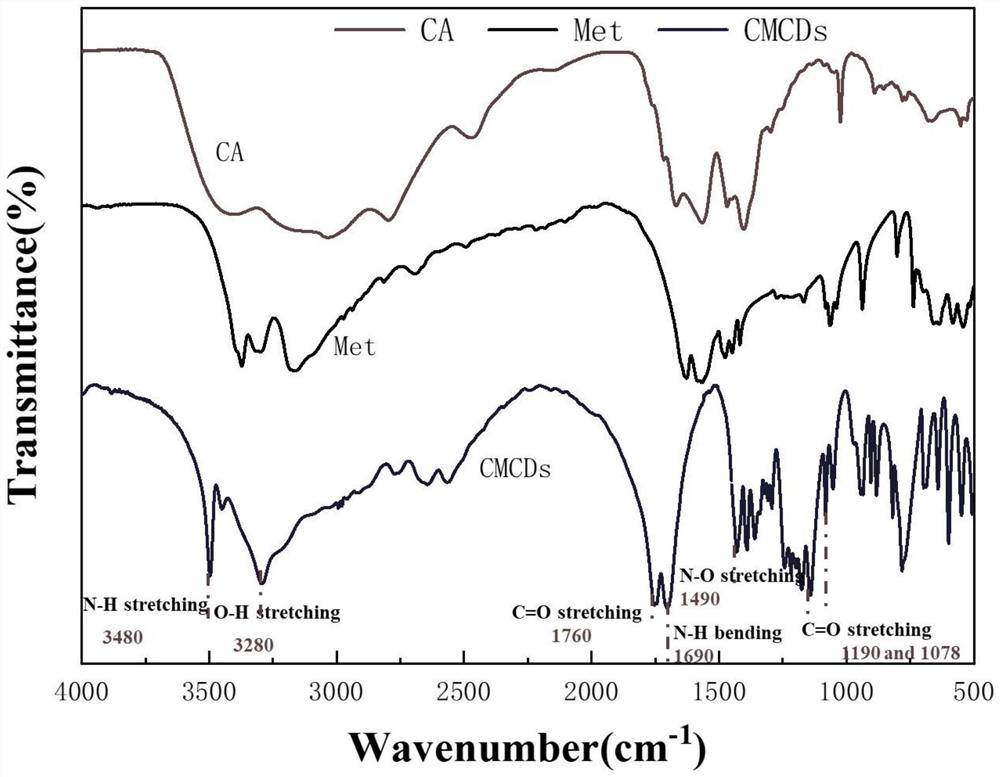
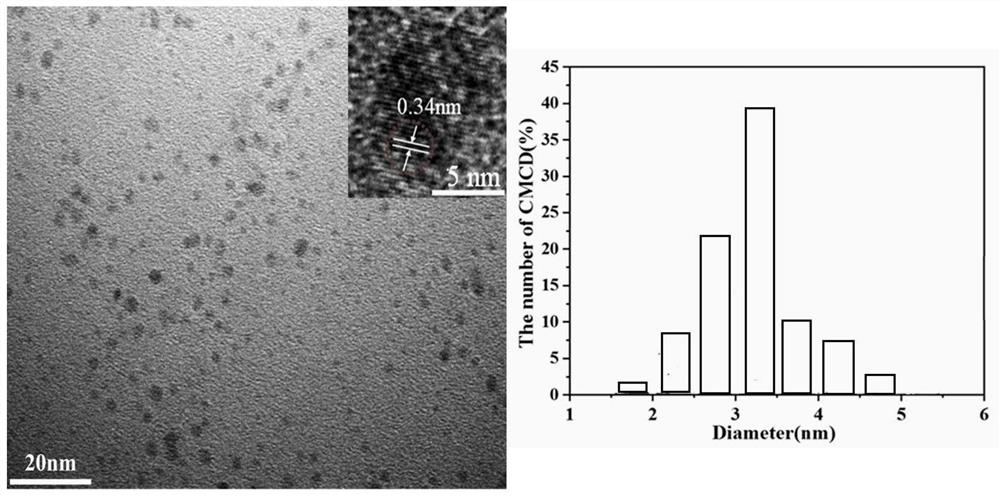
[0027] In one embodiment, a method for preparing metformin carbon dots is provided. The method includes the following steps: dissolving 33.124 mg of metformin hydrochloride (molecular weight=165.62 g / mol) and 0.1M citric acid (molecular weight=192.12 g / mol) in 10mL deionized water, ultrasonic for 10min;
[0028] Then CMCDs were synthesized by hydrothermal synthesis: the mixed solution of the two was transferred to a 50 mL autoclave system, and heated at 180 °C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com