Liquid core microcapsule based on hydrogel as well as preparation method and application of liquid core microcapsule
A technology of microcapsules and hydrogels, applied in the directions of microcapsule preparations, microsphere preparation, chemical instruments and methods, etc., can solve the problems of material, preparation, and maintenance cost increase, and achieve the effect of expanding the application field.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
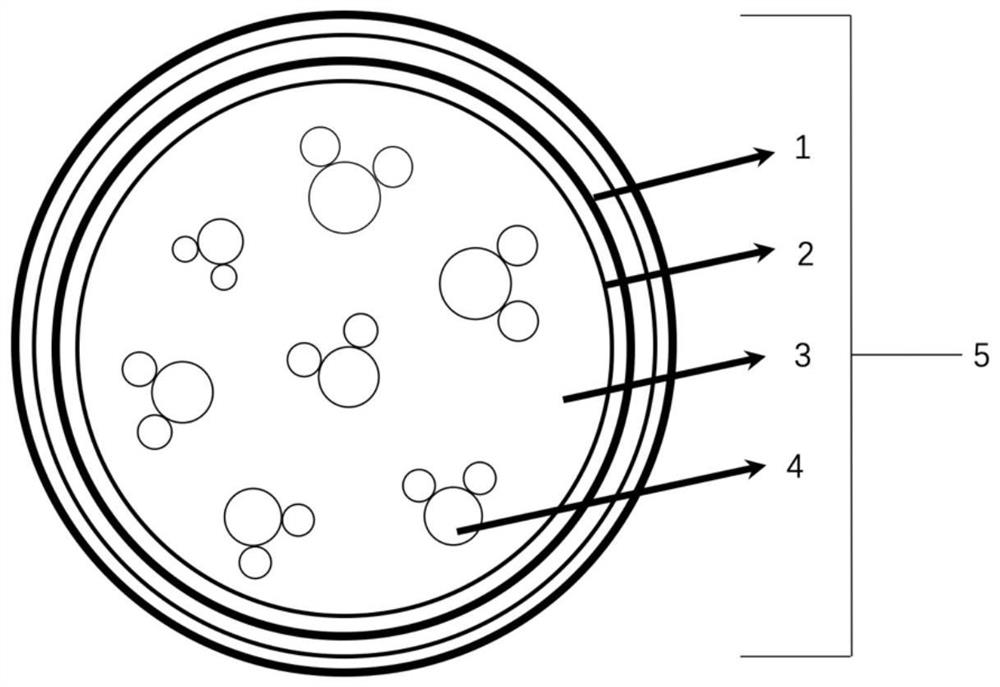
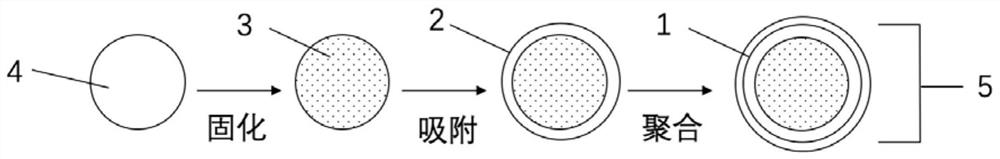
[0081] like figure 2 As shown, embodiments of the present invention also provide a method for preparing a hydrogel-based liquid-core microcapsule, comprising the following steps:
[0082] S1 mixes the conductive liquid and sodium alginate in a mass ratio of 100:0.5-1.5 to obtain a core material solution, which is ready for use;
[0083] S2 Mix urea and formaldehyde solution according to the mass ratio of 1:1 to 1:3, add deionized water to dilute, use triethanolamine solution pH=8.5, and then take out and cool to room temperature after magnetic stirring in a water bath for 30 to 60 minutes , to obtain a transparent urea-formaldehyde prepolymer wall material solution, which is ready for use; then, use electrostatic spray to drop the core material solution into a calcium chloride solution with a mass percentage concentration of 3% to 5% (for curing, forming a hydrogel stable system), the hydrogel microspheres are obtained after 20-30 minutes of complete curing, wherein by calcu...
Embodiment 1
[0114] 1. Preparation of liquid-core hydrogel microcapsules
[0115] First, mix 10 g of deionized water with 0.1 g of sodium alginate (concentration of 1.05-1.15 Pa.s) to obtain a core material solution, which is ready for use.
[0116] Then, mix 1 g of urea and 2 g of formaldehyde solution (37% by mass), and add 3 g of deionized water to dilute (adjust pH=8.5 with triethanolamine, then magnetically stir in a water bath (70° C., 300 rpm / min) for 30 After a few minutes, it was taken out and cooled to room temperature to obtain a transparent urea-formaldehyde prepolymer wall material solution, which was ready for use.
[0117] Next, the obtained core material solution was dropped into 60 g of calcium chloride solution (calcium salt solution) with a concentration of 4 wt % using electrostatic spray (10 kV), wherein a syringe pump (drop acceleration rate 50 mm / h) was used to push the syringe (Suzhou Jiaqicheng, 30G, 38mm, 0.16) formed into electrostatic spray droplets, and after ...
Embodiment 2
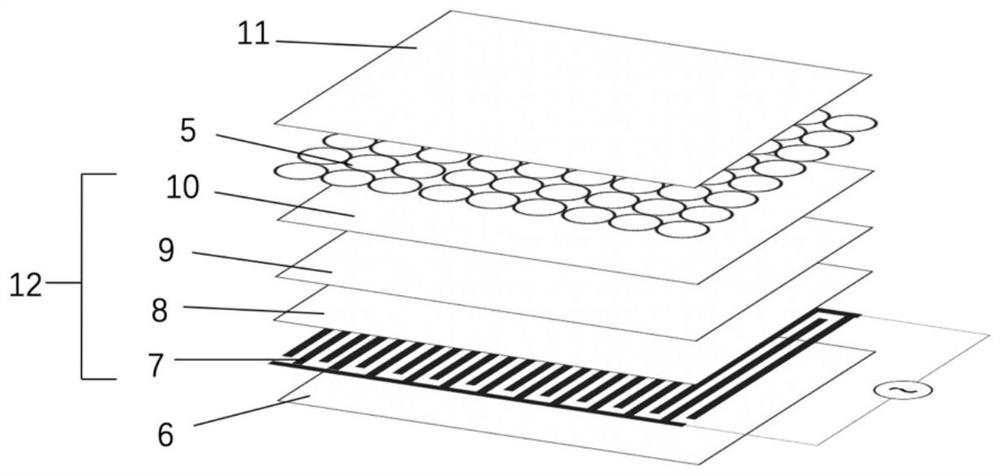
[0129] The difference between this example and Example 1 is that 10 g of deionized water in step 1 of this example is replaced by 10 g of polyethylene dioxythiophene-polystyrene sulfonate (PEDOT-PSS) dispersion liquid (containing PEDOT-PSS) 40 mg / L), wherein polystyrene sulfonate (PSS) is 1.5% of the mass of PEDOT-PSS. The remaining steps and parameters are the same as in Example 1.
[0130] The deformation rate of the hydrogel microspheres obtained in this example is 10%, and the appearance is mainly spherical, the average particle size is 796 μm, and the polymer dispersion index is 0.81.
[0131] The liquid-core microcapsule obtained in this example includes a polymer wall material and a core material wrapped inside the polymer wall material; the wall material is chitosan-urea-formaldehyde resin (CSUF), and the core material is hydrogel; the liquid core is The average particle size of the microcapsules was 798 μm, and the polymer dispersion index was 0.81. The chitosan-ure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com