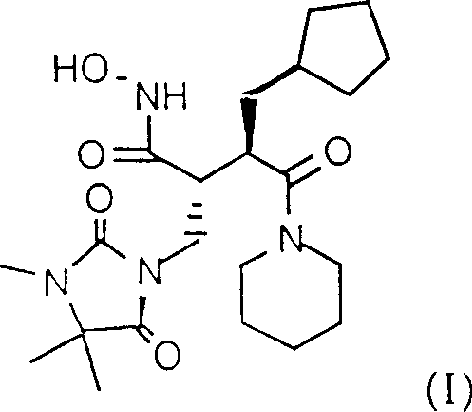
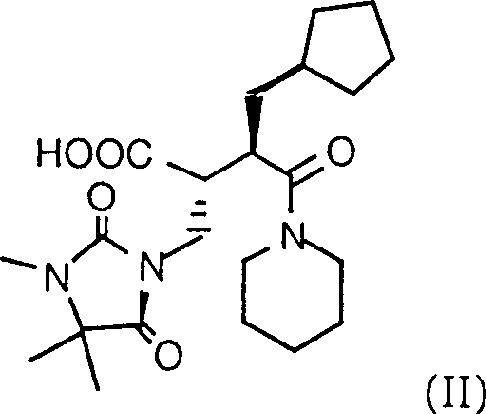
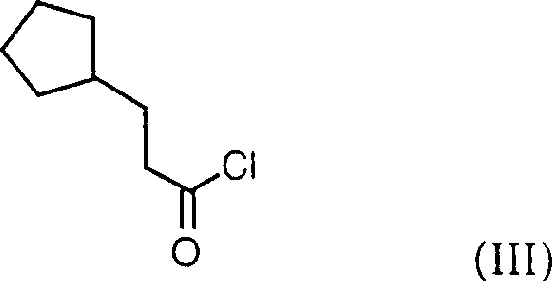
Prepn. of hydroxamic acid
A technology of carboxylic acid and hydroxylammonium, applied in the field of preparing hydroxamic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation of Hydroxyammonium Acetate
[0064] Place 100 g of hydroxylamine solution (50% in water) in a 500 ml flask and cool with an ice bath (0-5°) under magnetic stirring. While stirring, slowly add 93 g of glacialic acid for 30 minutes and cool. The reaction mixture was cooled to -20°C and the suspension was filtered. The crystals were washed with tert-butyl methyl ether and vacuum dried on a rotavap at 35°C to obtain 131 g (91%) of white crystals of hydroxylammonium acetate. m.p.: 87°.
Embodiment 2
[0066] In a 250 ml round-bottom flask, put 30 g of 1-[2(R)-[1(R)-carboxy-2-(3,4,4,-trimethyl-2,5-dioxo-1) -Imidazolidinyl)-ethyl]-3-cyclopentylpropionyl]piperidine and 8.71 g of N-hydroxy-pyridone were dissolved in 120 ml of dichloromethane. At room temperature, the mixture was treated with 10.73 g morpholino ethyl isocyanide. After 10-20 minutes, the mixture became clear and continued stirring at room temperature overnight. The above solution was slowly added to a suspension of 9.94 g of hydroxylammonium acetate and 7.2 g of triethylamine in 180 ml of dichloromethane, and the mixture was stirred for 4 hours. The reaction mixture contained 97% product and 3% starting material. The mixture was extracted with 95 ml of water. The aqueous layer was extracted with 60 ml of dichloromethane, and the combined organic layer was extracted with 95 ml of 5% NaHCO 3 Extract twice (total 190ml), and extract again with 95ml of 2% sulfuric acid solution. At 35-40°, the organic layer was evaporate...
Embodiment 3
[0068] a) According to the method described in Example 2, but using 10g 1-[2(R)-[1(R)-carboxy-2-(3,4,4,-trimethyl-2,5-dioxo) -1-imidazolidinyl)-ethyl]-3-cyclopentylpropionyl]piperidine is reacted with 2.9g N-hydroxy-pyridone and 3.66g morpholinoethyl isocyanide, followed by 2.47g hydroxylammonium Chloride reacts with 6g of triethylamine to obtain 50% dimers. These dimers further react with the product and starting materials. The final reaction mixture contains 55% product, 25% starting materials and 18%的dimer.
[0069] b) According to the similar method described in step a), the 1-[2(R)-[1(R)-carboxy-2-(3,4,4,-trimethyl-2,5-dioxo -1-imidazolidinyl)-ethyl]-3-cyclopentylpropionyl]piperidine is reacted with hydroxylammonium sulfate to obtain the same composition of the reaction mixture.
[0070] c) According to the similar method described in step a), add 1-[2(R)-[1(R)-carboxy-2-(3,4,4,-trimethyl-2,5-dioxo -1-imidazolidinyl)-ethyl]-3-cyclopentylpropionyl]piperidine and hydroxylammoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com