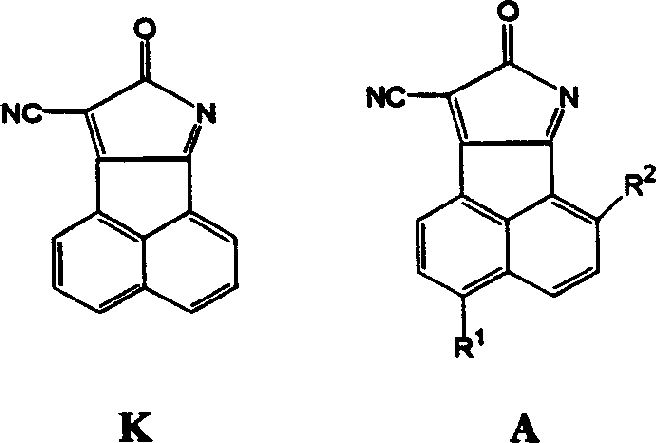
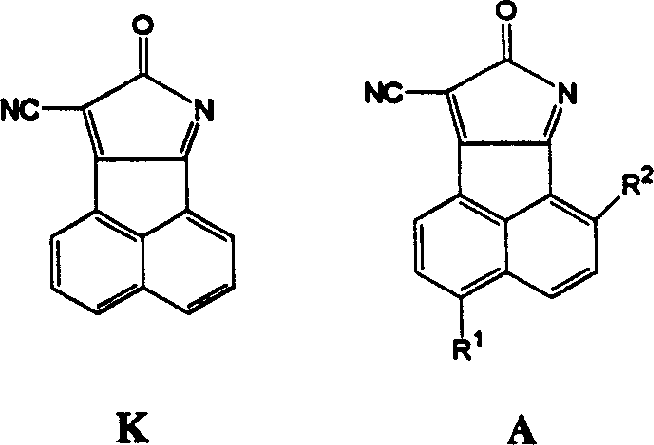
8-oxy-8h acenaphthene (1,2-6) pyrrol-9 nitrile fluorescence chromophore and its derivative
A chromophore and derivative technology, which is applied to 8-oxy-8H acenaphthene (1), can solve the problems of reducing the sensitivity of the probe and achieve the effect of high fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
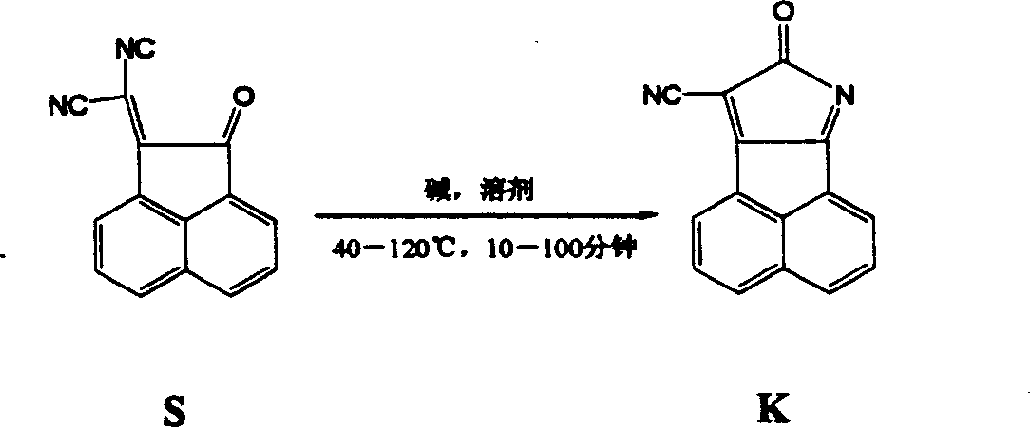
[0032]
[0033] 1 g of 2-(2-oxo-2H-acenaphthyl)malononitrile, 0.2 g of potassium carbonate, and 10 ml of dimethyl sulfoxide were heated to 100°C, stirred for 15 minutes, and cooled to precipitate crystals. After filtering, washing and drying with water, the product 8-oxo-8Hacenaphtho(1,2-b)pyrrole-9-carbonitrile was obtained as a yellow-brown crystal with a yield of 99%.
[0034] 1 H NMR (400M, DMSO): δ8.71-8.69 (d, J=8.0Hz, 1H), 8.67-8.65 (d, J=7.6Hz, 1H), 8.64-8.62 (d, J=8.0Hz, 1H ), 8.42-8.40(d, J=7.6Hz, 1H), 8.04-8.08(t, J=8.0Hz, 1H), 7.99-7.95(t, J=7.8Hz, 1H); 13C NMR (100M, DMSO): δ177.48, 138.26, 137.73, 134.40, 132.72, 131.82, 131.37, 128.91, 127.94, 127.37, 126.13, 122.22, 119.72, 113.82, 113.38; IR (KBr) cm -1 : 2231, 1643, 1577; ESI-MS: M+Na + (253, m / z).
Embodiment 2
[0036]
[0037] Add 0.32 g of butylamine to 50 ml of acetonitrile solution of 1 g of 8-oxo-8H acenaphtho(1,2-b)pyrrole-9-carbonitrile, stir at room temperature for 30 minutes, evaporate part of the solvent, and precipitate the product 3-butylamino 8- Oxy-8Hacenaphtho(1,2-b)pyrrole-9-carbonitrile Al. Yield 70%.
[0038] 1 H NMR (400M, DMSO): δ9.604 (br s, -NH-, 1H), 8.95-8.93 (d, J=7.6Hz, 1H), 8.60-8.58 (d, J=7.2Hz, 1H), 7.98-7.96(d, J=8.8Hz, 1H), 7.88-7.92(t, J=7.8Hz, 1H), 7.04-7.02(d, J=9.2Hz, 1H), 3.60-3.59(br s,- NHCH 2 CH 2 -, 2H0, 1.75-1.71 (m, -NHCH 2 CH 2 CH 2 -, 2H), 1.43-1.47 (m, -CH 2 CH 2 CH 2 CH 3 ), 0.94-0.98 (t, J=7.2Hz, -CH 2 CH 3 );IR(KBr)cm -1 : 3284, 2217, 1619, 1562, 1529; ESI-MS: [M-H] - (300m / z).
Embodiment 3
[0040]
[0041] Add 0.43 g of cyclohexylamine to 50 ml of acetonitrile solution of 1 gram of 8-oxo-8H acenaphtho(1,2-b)pyrrole-9-carbonitrile, stir at room temperature for 30 minutes, evaporate part of the solvent, and precipitate the product 3-cyclohexylamino -8-Oxo-8Hacenaphtho(1,2-b)pyrrole-9-carbonitrile A2. Yield 68%.
[0042] 1 H NMR (400M, DMSO): δ9.13-9.11 (d, J = 7.6Hz, -NH-, 1H) 9.03-9.01 (d, J = 7.6Hz, 1H), 8.54-8.52 (d, J = 7.2 Hz, 1H), 7.90-7.87(d, J=9.2Hz, 1H), 7.87-7.83(t, J=8.0Hz, 1H), 7.09-7.07(d, J=9.2Hz, 1H), 3.90(br s, -NHCH-, 1H), 2.05-2.02 (br d, -NHCH (CH 2 * CH 2 ) 2 , CH 2 , 2H), 1.85-1.82 (br d, -NHCH (CH 2 * CH 2 ) 2 CH 2 , 2H), 1.72-1.69 (brd, -NHCH (CH 2 CH 2 ) 2 CH 2 * , 1H), 1.43-1.58 (m, -NHCH (CH 2 CH 2 ) 2 CH 2 , 4H), 1.19-1.23 (brd, -NHCH (CH 2 CH 2 ) 2 CH 2 * , 1H); IR(KBr)cm -1 : 3324, 2215, 1631, 1575, 1492; ESI-MS: [M+H] + (328, m / z).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com