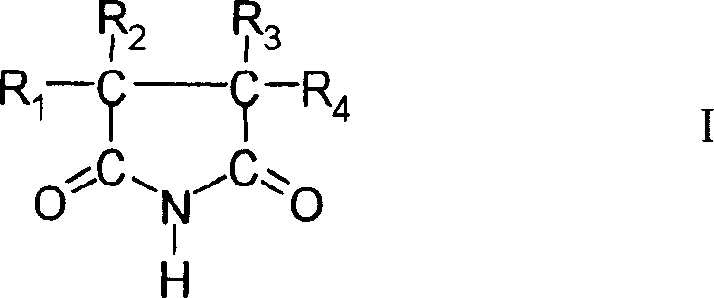
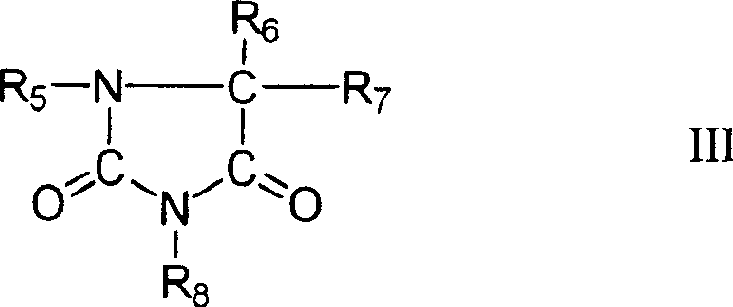
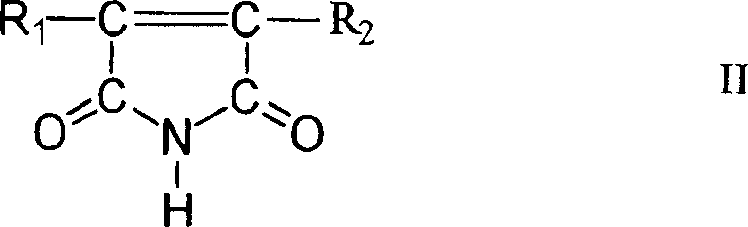Cyanide-free monovalent copper eletroplating solutions
A technology of electroplating solution and divalent copper ions, which is applied in the field of copper electroplating on a substrate, a special complexing agent, and can solve the occasional instability of the plating solution and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The following compounds were dissolved in deionized water to prepare a monovalent copper electroplating solution.
[0039] 5,5-Dimethylhydantoin 90g / l
[0040] Cuprous chloride 15g / l
[0041] Sodium bisulfite 30g / l
[0042] Triethylenetetramine 0.05ml / l
[0043] Adjust the pH of the plating solution to 8.5 with sodium hydroxide. Maintain the temperature of the plating solution at 110-125°F (43-52°C), and use the motor to drive the stirrer to stir the plating solution.
[0044] In the resulting bath, with 5 and 10A / ft 2 (0.54 and 1.08A / dm 2 ) Cathode current density Electroplating the brass test piece and steel test piece until the thickness is 0.3 mil (7.5 μm). The current density is 0.54A / dm 2 (5A / ft 2 ), the plating time is 48 minutes, and the current density is 1.08A / dm 2 (1.0A / ft 2 ), the plating time is 24 minutes. The deposited copper combines well with the base metal and has a bright appearance.
Embodiment 2
[0046] Except for 27 g / l of copper chloride as the source of copper ions, the rest were all prepared as described in Example 1 to prepare a monovalent copper electroplating solution. As described in Example 1, the brass test piece and the steel test piece were electroplated. The appearance and bonding force of the plated copper layer are basically the same as in Example 1.
Embodiment 3
[0048] Except that 15 g / l cuprous oxide was used as the source of copper ions, the others were all as described in Example 1 to prepare a monovalent copper electroplating solution. As described in Example 1, the brass test piece and the steel test piece were electroplated. The appearance and bonding force of the plated copper layer are basically the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com