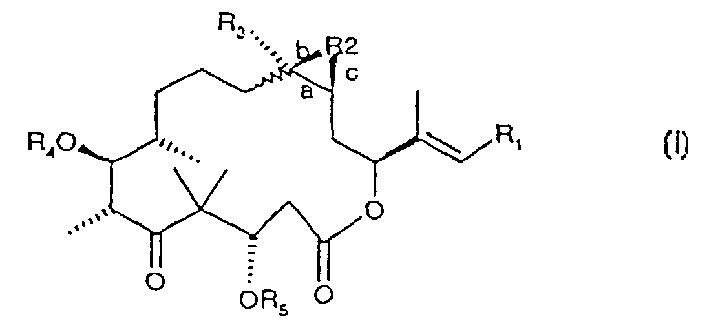
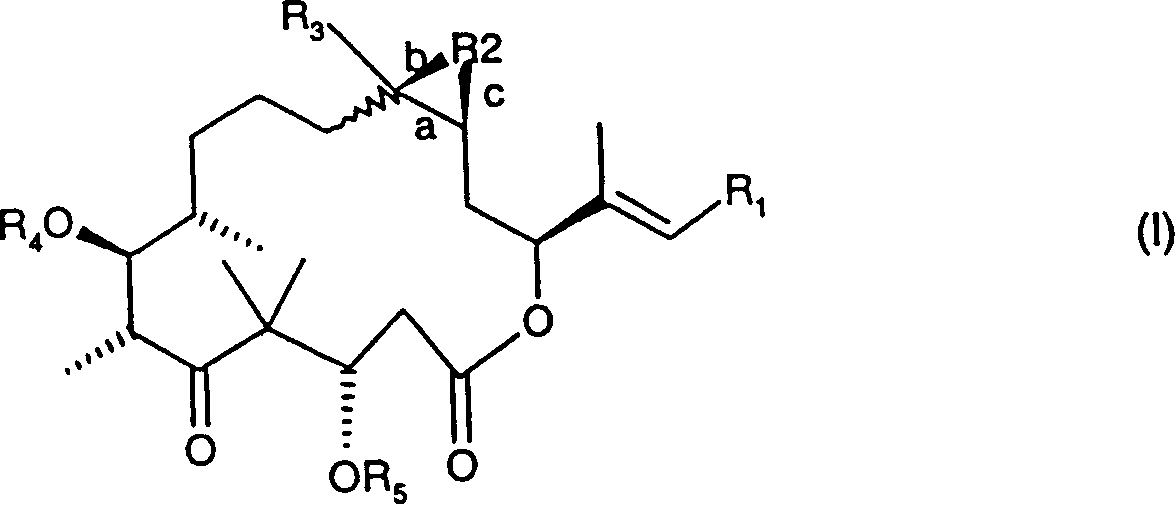
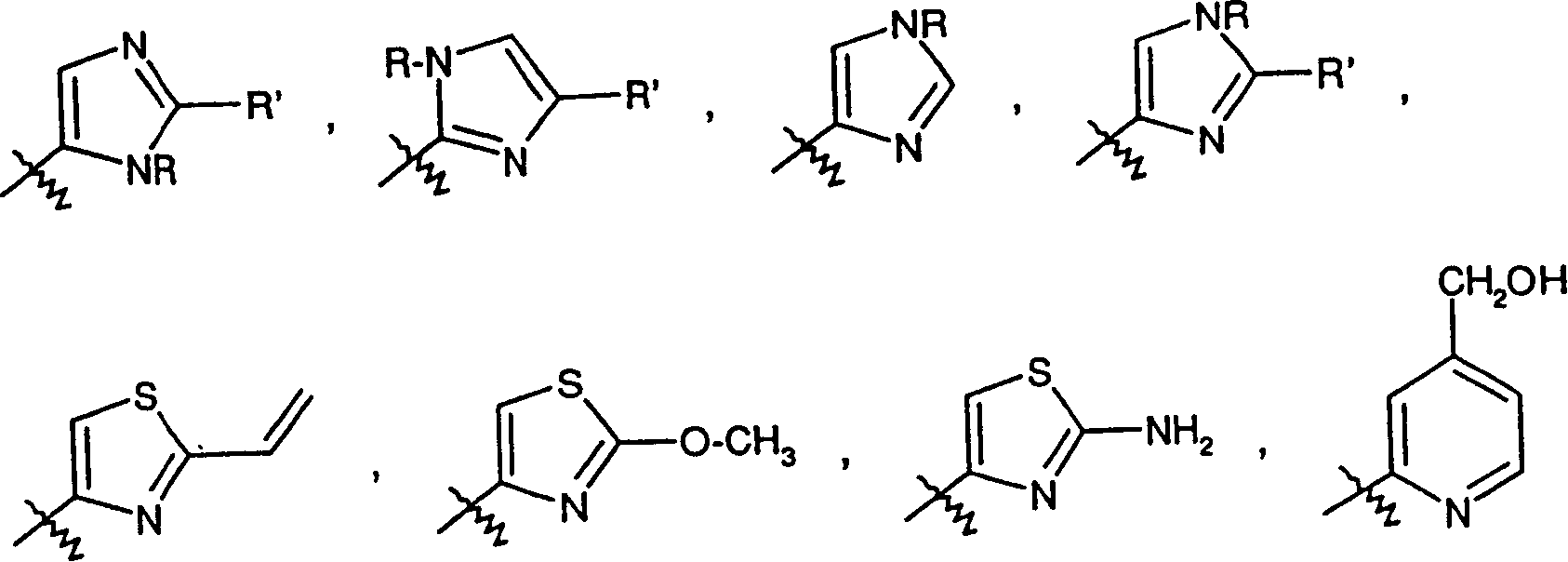
Epothilones derivatives, their synthesis and uses
A compound, single bond technology, applied in the field of epsilon analogs, can solve the problem of unsuitable coupling of 24 with vinylstannane 8q
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0237] Example 1: 5-Methylpyridine Analog D of Epsilon B
[0238] Process 1B
[0239]
[0240] To a solution of B (20 mg, 0.035 mmol) in degassed dimethylformamide (=DMF; 350 μl, 0.1 M) was added C (17 mg, 0.077 mmol, 2.1 eq) followed by AsPh 3 (4 mg, 0.4 equiv), PdCl 2 (MeCN) 2(2 mg, 0.2 equiv) and CuI (1 mg, 0.1 equiv), and the resulting slurry was placed in an oil bath at 80-85°C for 25 minutes. The reaction mixture was then cooled to room temperature and DMF was distilled off. The residue was dissolved in ethyl acetate and filtered through a small plug of silica eluting with hexane / ethyl acetate (1 / 1, v / v). The solution was then concentrated in vacuo and purified by preparative TLC (hexanes / ethyl acetate 1 / 2) to give compound D: MS (electrospray): Expected: (M+H) + =502, measured: 502.
[0241] 1 H-NMR (600MHz, CDCl 3 ): 8.37 (s, 1H, pyridine H); 7.50 (d, l = 7,5Hz, 1H, pyridine H); 7.19 (d, l = 7.5Hz, 1H, pyridine H), 6.59 (s, 1H, = CH pyridine).
[0242] raw...
Embodiment 2
[0246] Example 2: Analogously to Example 1, the following examples were prepared:
[0247]
[0248]
Embodiment 3
[0249] Example 3: Total Synthesis of Epsilon E and Related Side Chain Modified Analogs by Stille Coupling Strategy
[0250] The first general synthesis of epsilone E(3) was accomplished via a strategy in which the key step was the Stille coupling between vinyl iodide 7 and the thiazole moiety (8h, Scheme 2a) (Stille et al. "International English Edition of Applied Chemistry" 1986, 25, 508-524; Farina et al. "Journal of Organic Reactions" 1997, 50, 1-65). The macrolactone core fragment 7 prepared by ring-closing olefin displacement (RCM) was then used to provide suitable and feasible various side-chain modified epsilon analogs (9) for biological evaluation ( Process 2b). The RCM reaction used to obtain 7 also provided the trans-macrolactone (11, Scheme 2b), which served as an alternative template for the Stille coupling process, providing an alternative arrangement of the analog 10.
[0251] Process 2:
[0252] a) Retrosynthetic analysis and strategy for the total synthesis ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com