Preparation method of regenerated polyester fibers
A technology for regenerated polyester and fiber, applied in the preparation of ester groups and hydroxyl groups, chemical instruments and methods, single-component polyester artificial filaments, etc., can solve the problem of affecting the quality of repolymerization products, poor quality, low catalytic efficiency, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
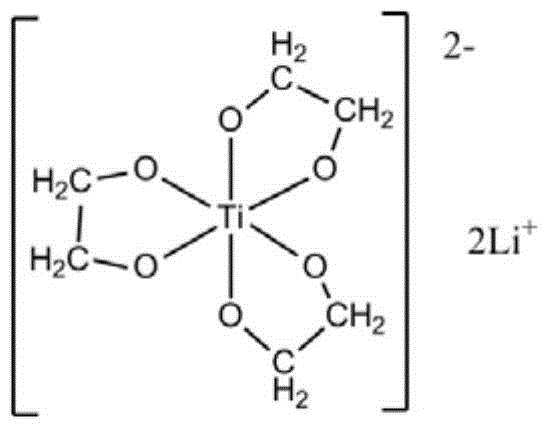
[0054] At first carry out the preparation of ethylene glycol lithium titanate, it comprises the following steps:
[0055] a) Uniformly mix tetraethyl titanate with ethylene glycol at 50°C under nitrogen protection, the molar ratio of tetraethyl titanate to ethylene glycol is 1:100;
[0056] b) Add lithium hydroxide, the molar ratio of lithium hydroxide to tetraethyl titanate is 2.05:1, continue to stir until the reaction system is a uniform and transparent liquid, then raise the reaction temperature to 180°C;
[0057] c) During the reaction process, reflux ethylene glycol and exclude the low-boiling ethanol and water generated by the reaction. The reaction time is 3 hours. Completely evaporate the ethylene glycol in the system, and completely evaporate the dihydric alcohol in the system to obtain a white solid, which is recrystallized three times with ethanol-chloroform to obtain a colorless crystal, which is to obtain ethylene glycol lithium titanate;
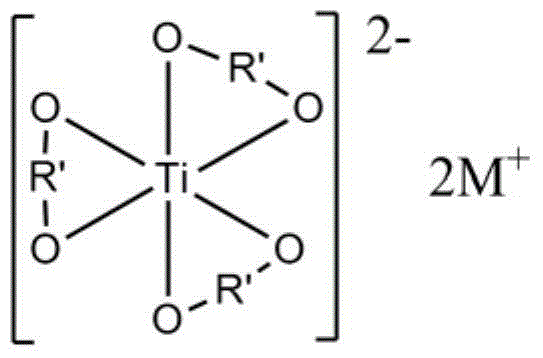
[0058] The structural...
Embodiment 2
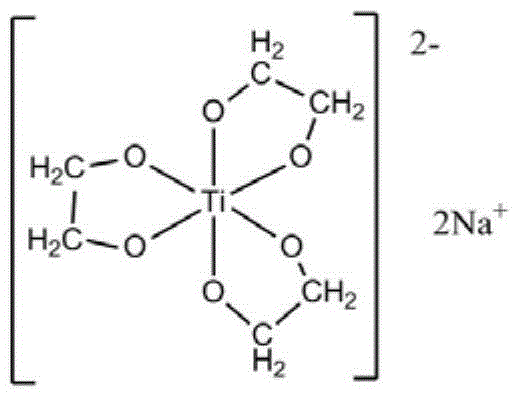
[0069] At first carry out the preparation of ethylene glycol sodium titanate, it may further comprise the steps:
[0070] a) Uniformly mix tetra-n-butyl titanate with ethylene glycol at 150°C under nitrogen protection, the molar ratio of tetra-n-butyl titanate to ethylene glycol is 1:50;
[0071] b) Add sodium hydroxide, the molar ratio of sodium hydroxide to tetra-n-butyl titanate is 2.03:1, and continue to stir until the reaction system becomes a uniform and transparent liquid, then raise the reaction temperature to 210°C;
[0072] c) During the reaction process, reflux ethylene glycol and exclude the low-boiling n-butanol and water generated by the reaction. The reaction time is 2 hours. Completely evaporate the ethylene glycol in the system and completely evaporate the dihydric alcohol in the system to obtain a white solid, which is recrystallized three times with ethanol-chloroform to obtain a colorless crystal, which is to obtain ethylene glycol sodium titanate;
[0073...
Embodiment 3
[0084] At first carry out the preparation of propylene glycol potassium titanate, it may further comprise the steps:
[0085] a) Uniformly mix tetraisopropyl titanate with 1,2-propanediol at 100°C under nitrogen protection conditions, the molar ratio of tetraisopropyl titanate to 1,2-propanediol is 1:80;
[0086] b) Add potassium hydroxide, the molar ratio of potassium hydroxide to tetraisopropyl titanate is 2.02:1, continue to stir until the reaction system is a uniform and transparent liquid, then raise the reaction temperature to 200°C;
[0087] c) During the reaction, reflux 1,2-propanediol and exclude the low-boiling isopropanol and water generated by the reaction. The reaction time is 4 hours. After the reaction is completed, the temperature is 200 ° C, and the reaction system pressure is 0.7 atm, 1 min Completely evaporate the ethylene glycol in the system, and completely evaporate the glycol in the system to obtain a light yellow solid, which is recrystallized three ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com