Novel optically active aminopentane derivative
A technology of propylaminopentane and compounds, applied in the field of new optically active aminopentane derivatives, can solve the problem that the optical separation of racemic compounds is not so easy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
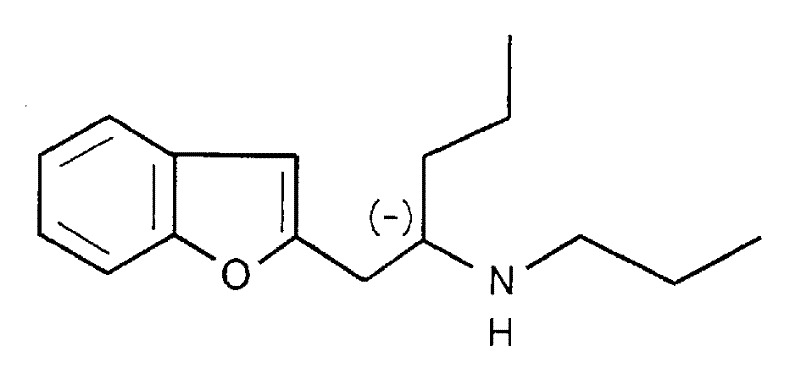
[0018] Preparation of (-)-1-(benzofuran-2-yl)-2-propylaminopentane hydrochloride
[0019] Use the HPLC method to implement optical separation under the following conditions
[0020] 115 μl of 8 mg / ml (±)-1-(benzofuran-2-yl)-2-propylaminopentane hydrochloride (racemate) was injected directly into the HPLC.
[0021] HPLC: LC-6AD, Shimadzu, Kyoto, Japan.
[0022] Column: CHIRALPACK AD 20 mm φ x 250 mm (DAICEL)
[0023] Mobile phase: hexane: isopropanol: trifluoroacetic acid = 100: 2: 0.1
[0024] Flow rate: 120ml / min
[0025] Detector: SPD-10A UV / VIS detector (280 nm), Shimadzu, Kyoto, Japan
[0026] Humidity: room temperature
[0027] Melt oily (-)-1-(benzofuran-2-yl)-2-propylaminopentane in anhydrous ether, then add hydrogen chloride to the ether solution to make it saturated, thus forming hydrochloride .
[0028] Melting point: 165.0-166.0°C
[0029] lR: 3425, 2970, 2870, 2780, 2735, 2690, 2520, 2430, 1605,
[0030] 1590, 1472, 1455, 1255, 1167, 942, 805, 770, 760 cm ...
Embodiment 2
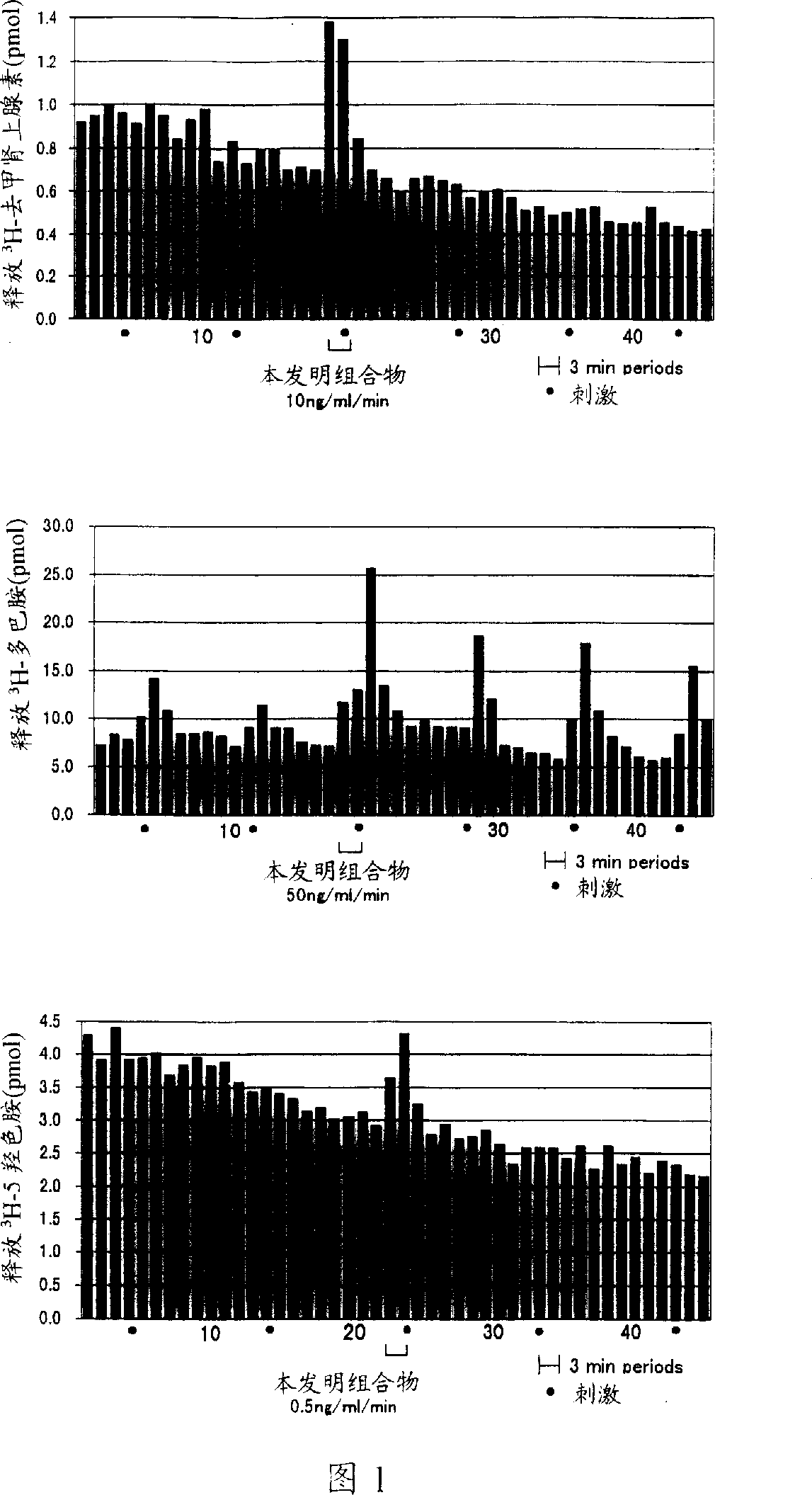
[0049] Determination of biogenic amines released from the brainstem of test rats by electrical stimulation
[0050] This method has been described in "Life Science" (Life Sci.) 58, 2101-2114 (1996), in which male test rats weighing 280-350 grams were slapped on the back of the head to stun them, and then removed Its brainstem (average weight about 800 mg) was immersed in oxygenated (95% O 2 with 5% CO 2 ) in Krebs' solution at 37°C for 30 minutes. Then, put 20 µl of 3 H-norepinephrine solution (1-[7,8- 3 H]-norepinephrine; specific activity: 30-50 Ci / mmol; Amersham) was added to the test preparation and absorbed for 45 minutes. The composition of Krebs solution is [mmol / L]: Na + 137.4;K + 5.9, Ca 2+ 2.5, Mg 2+ 1.2, Cl - 120.2,H 2 PO - 4 1.2, HCO 3 - 25.0, SO 1.2, glucose 11.5, ascorbic acid 0.3, EDTA-2Na 0.03. Bajiline (12 mmol) was added to Krebs' solution to inhibit MAO activity during the uptake of the labeled mediator.
[0051] absorbing 3 After H-norepi...
Embodiment 3
[0056] Conditional Avoidance Job Effects in Shuttle Boxes
[0057] This method is described in Life Sci. 58.817-827 (1996). Conditioned avoidance reflex effects (CARs) were analyzed in a shuttle box using male and female test rats (weight 200-220 g) whose learning ability was reduced by administration of tetrabenazine. The device described therein according to Psychopharmacologia Journal, 10, 1-5 (1996) constitutes the apparatus used in the experiments. Wherein the above-mentioned male and female test rats (200-220 gram weight / only) are trained to pass through obstacles under the conditions of conditioned stimulus [CS (control signal), light flash and buzzing sound]. If they refused to do so, they were punished by electrical stimulation of their paws (1 mA), here unconditioned (US). If the rat did not respond to the above US within 5 seconds, it was considered an escape failure (EF). In addition, behavior that is not related to this condition is considered an inter-signal r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com