Production process of heavy soda ash
A technology of heavy soda ash and production method, applied in chemical instruments and methods, alkali metal compounds, alkali metal carbonates, etc., can solve the problems of high manufacturing cost, short service life of crystallizer, difficult control of salt content and other impurities, etc. problems, to achieve the effect of low salt and other impurities, bright particle surface, easy production control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
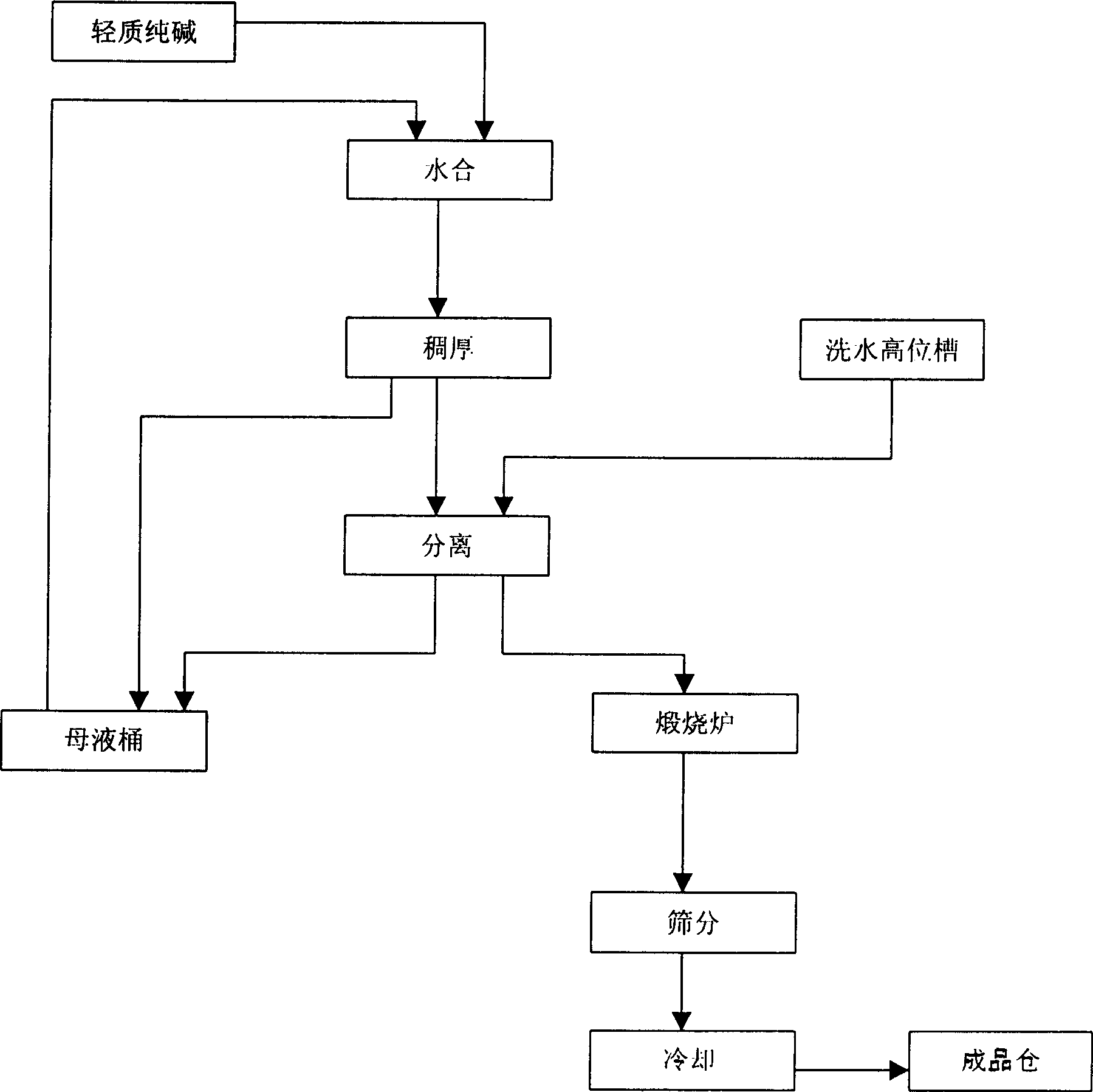
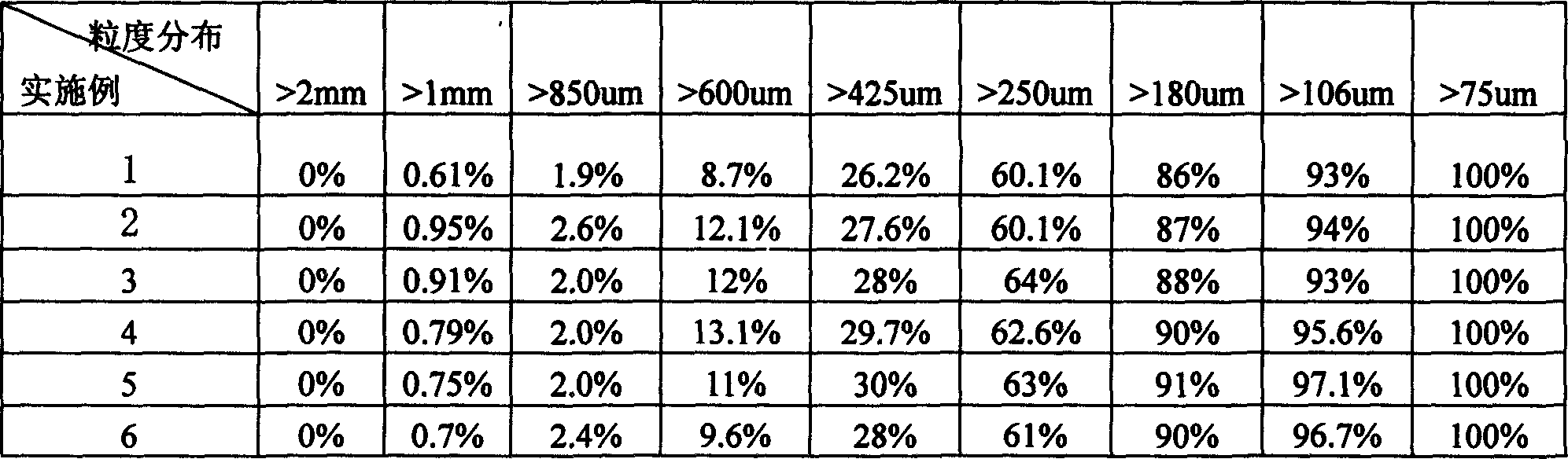
[0014] Add light soda ash and saturated sodium carbonate solution with a weight ratio of 1:0.7 into the hydration crystallizer, and carry out hydration reaction at a temperature of 95° C. for 5 minutes. The solid-liquid volume ratio of the crystal slurry after hydration is 25%, and the crystal slurry after the hydration reaction is sent to a thickener for thickening until the solid-liquid volume ratio is 35%, and then enters the centrifuge for dehydration. The washing water from the water head tank is used to wash the filter cake in the centrifuge, and the washing water volume is 230kg / t 重质纯碱 Wash away the salt and other impurities in the monohydrate alkali, control the free water content of the dehydrated monohydrate alkali at 2.8%, and finally enter the heavy soda ash furnace for calcination, and carry out particle size screening and cooling after the furnace to obtain low salt weight quality soda ash. Heavy soda ash has a uniform particle size as crystals (see Table 1 for ...
Embodiment 2
[0016] Add light soda ash and saturated sodium carbonate solution with a weight ratio of 1:0.8 into the hydration crystallizer, and carry out hydration reaction at a temperature of 105° C. for 8 minutes. The solid-liquid volume ratio of the crystal slurry after hydration is 30%, and the crystal slurry after the hydration reaction is sent to a thickener for thickening until the solid-liquid volume ratio is 40%, and then enters the centrifuge for dehydration. The washing water from the water head tank is used to wash the filter cake in the centrifuge, and the washing water volume is 240kg / t 重质纯碱 Wash away the salt and other impurities in the monohydrate alkali, control the free water content of the dehydrated monohydrate alkali at 2.5%, and finally enter the heavy soda ash furnace for calcination. quality soda ash. The particle size of heavy soda ash is uniform as crystals (the particle size distribution is shown in Table 1), and the service life of the hydration crystallizer i...
Embodiment 3
[0018] Add light soda ash and saturated sodium carbonate solution with a weight ratio of 1:1 into the hydration crystallizer, and carry out hydration reaction at a temperature of 100° C. for 10 minutes. The solid-liquid volume ratio of the crystal slurry after hydration is 35%, and the crystal slurry after the hydration reaction is sent to a thickener for thickening until the solid-liquid volume ratio is 45%, and then enters the centrifuge for dehydration. The washing water from the water head tank is used to wash the filter cake in the centrifuge, and the washing water volume is 245kg / t 重质纯碱 Wash away the salt and other impurities in the monohydrate alkali, control the free water content of the dehydrated monohydrate alkali at 1.5%, and finally enter the heavy soda ash furnace for calcination. quality soda ash. Heavy soda ash has a uniform particle size as crystals (see Table 1 for its particle size distribution), and the service life of the hydration crystallizer is 8 days....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com