Dehydrogenation method of CO2 raw material gas for synthesizing urea
A carbon dioxide, raw gas technology, applied in chemical instruments and methods, preparation of organic compounds, inorganic chemistry, etc., can solve problems such as unusability, and achieve the effect of eliminating explosions, eliminating explosion hazards, and solving potential safety hazards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
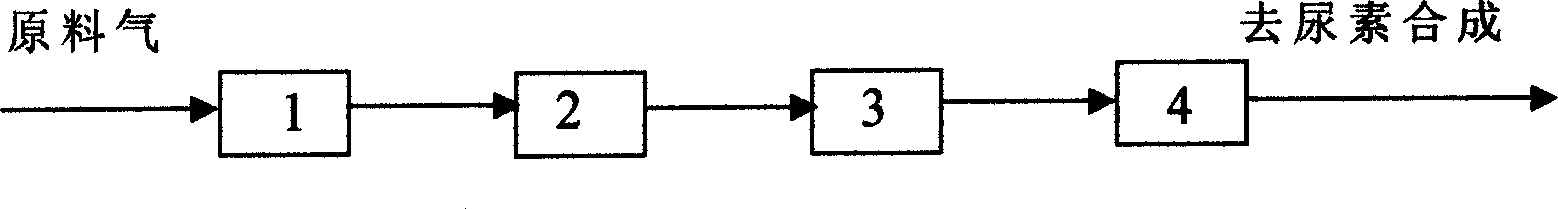
[0018] A certain nitrogenous fertilizer plant uses coal as raw material, and when the urea production capacity of expansion and transformation reaches 200,000 tons / year, the CO2 raw material gas dehydrogenation method for urea synthesis of the present invention is adopted, and its process flow is shown in the attached figure 1 As shown, when the inlet temperature of the TH-2 dehydrogenation catalyst is at 140-160°C, the H2 content can be removed from 0.3-0.6% to 50-300ppm, and a satisfactory effect has been achieved. Table 1 lists five operations Monthly and 10-month data.
[0019] Table 1 Data of urea dehydrogenation new technology running for 5 and 10 months in 2000
[0020] Date (May)
Outlet temperature °C
Date (October)
Outlet temperature °C
1
140-143
160-165
1
150-155
170-175
3
140-149
160-167
3
150-153
170-174
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com