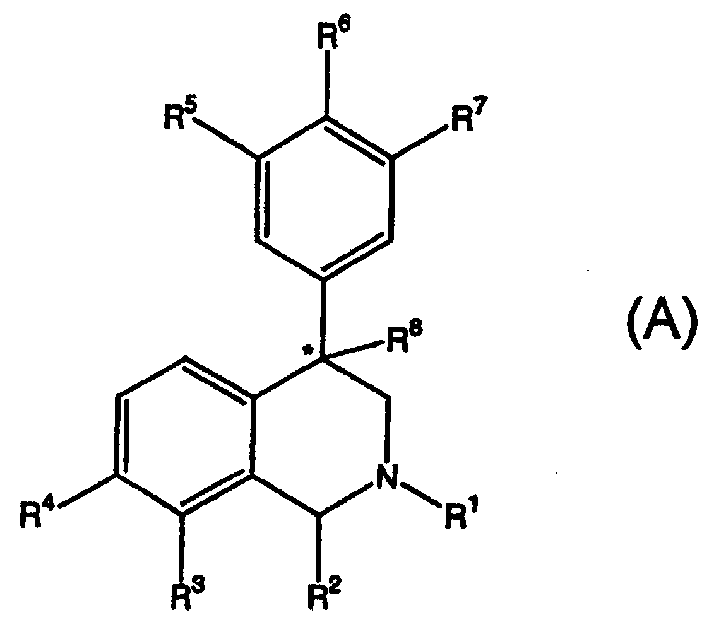
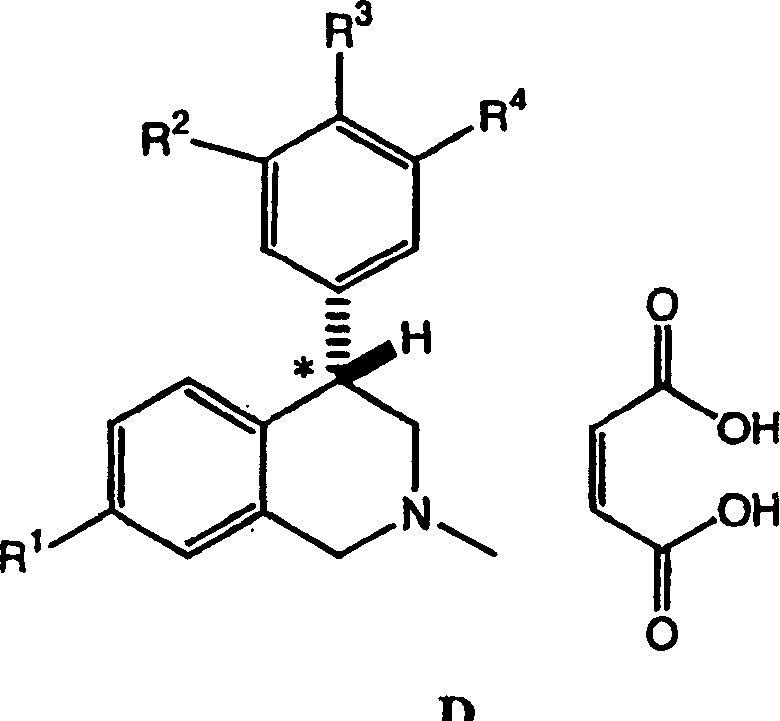
4-phenyl-substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine and serotonin
A phenyl and alkyl technology, applied in the direction of medical preparations containing active ingredients, metabolic diseases, preparations for in vivo tests, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0416] The preparation of embodiment 12,7-dimethyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline
[0417]Step A: m-tolualdehyde (500 mg, 4.16 mmol), D-(methylaminomethyl ) A solution of benzyl alcohol (630mg, 4.16mmol) and acetic acid (0.5ml) was stirred in methanol (16ml). The reaction mixture was stirred for 5 minutes at 0°C and for two days at ambient temperature. The reaction mixture was adjusted to pH 12 with 2N sodium hydroxide, diluted with water and extracted with ether (3x). The combined organic extracts were washed with brine, dried over anhydrous magnesium sulfate and the solvent was removed in vacuo to give the desired intermediate (1.24 g): 1 HNMR (300MHz, CDCl 3 )δ7.08-7.35(m, 9H), 4.73-4.77(m, 1H), 3.71(d, J=13.0Hz, 1H), 3.50(d, J=13.0Hz, 1H), 2.46-2.67(m , 2H), 2.36(s, 3H), 2.32(s, 3H); CI MS m / z=256[C 17 h 21 NO+H] + .
[0418] Step B: The product from Step A (1.24g, 4.90mmol) was stirred in dichloromethane (208ml) and treated with concentrated sulfuric acid...
Embodiment 42
[0420] Example 42, Preparation of 7-dimethyl-4-(3-fluorophenyl)-1,2,3,4-tetrahydroisoquinoline
[0421] Step A: m-Tolualdehyde (1.66g, 14.0mmol) was treated with methylamine (40% in water, 1.39ml, 18.0mmol) in methanol (20ml) at room temperature. The reaction was stirred for 20 minutes and treated with sodium borohydride (0.26 g, 7.0 mmol) in portions. The reaction was stirred for 1 hour and treated with 3'-fluoro-2-bromoacetophenone (3.0 g, 14.0 mmol), then stirred at room temperature for 45 minutes. Finally, the reaction was treated in portions with sodium borohydride (0.52 g, 14.0 mmol) and stirring was continued overnight. The reaction was diluted with water (100ml) and extracted with dichloromethane (3 x 100ml). The combined organic extracts were washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. Purification by column chromatography on silica gel, eluting with hexane / ethyl acetate (3 / 1 ), afforded the aminoalcohol (4.3 g) ...
Embodiment 62
[0424] Example 62, Preparation of 7-dimethyl-4-(4-fluoro-3-methylphenyl)-1,2,3,4-tetrahydroisoquinoline
[0425] Step A: m-Tolualdehyde (4.0 g, 33.0 mmol) was treated with methylamine (40% in water, 3.36 mL, 43.0 mmol) in methanol (40 mL) at room temperature. The reaction was stirred for 20 minutes and treated with sodium borohydride (0.64 g, 33.0 mmol) in portions. The reaction was stirred for 1 hour and treated with 4'-fluoro-3'-methyl-2-bromoacetophenone (7.69 g, 33.0 mmol), then stirred at room temperature for 45 minutes. Finally, the reaction was treated in portions with sodium borohydride (1.0 g, 33 mmol) and stirring was continued overnight. The reaction was diluted with water (100ml) and extracted with dichloromethane (3 x 100ml). The combined organic extracts were washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. Purification by column chromatography on silica gel, eluting with hexane / ethyl acetate (2 / 1), afforded...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com