Method of hydrogen generation for fuel cell applications and a hydrogen-generating system
A device-in-device and generator technology that is applied in the field of hydrogen generators and can solve problems such as restrictions, icing, and danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
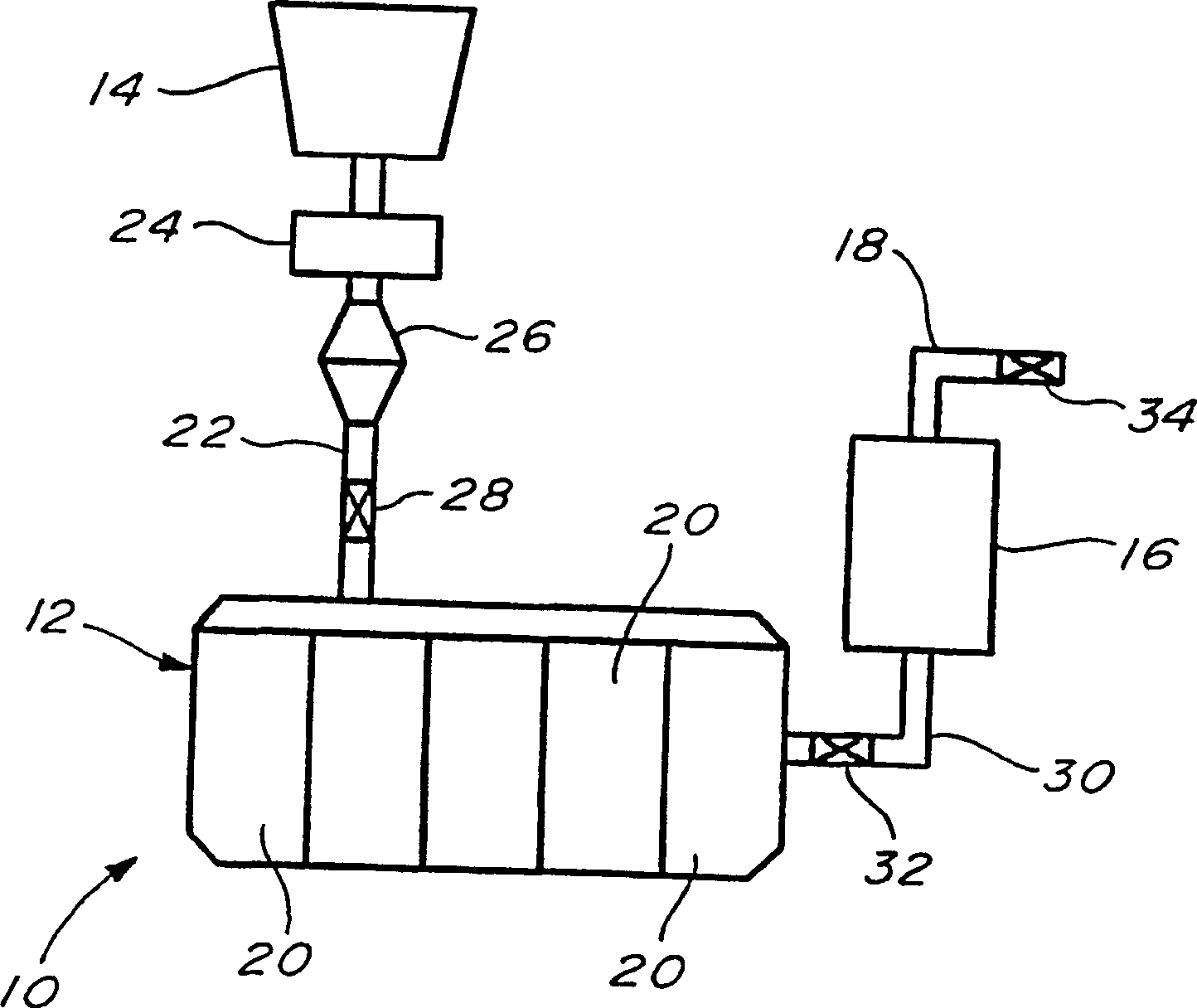
[0021] The present invention relates to a new method of generating hydrogen, especially for a fuel cell device. In this method, hydrogen is generated by the chemical reaction of metal hydrides with alcohols. The metal hydride may be a simple metal hydride or a complex metal hydride. When it is a simple metal hydride, the reaction proceeds as the basic reaction of hydrogen generation by the following reaction:
[0022]
[0023] Among them, MH x Is a simple metal hydride, ROH is an alcohol. In the above metal hydrides, M can be, for example, such as Li, Na, K, Mg, Ca, Be, Sr, K, Nb, Zr or Ti; R can be an alkyl group of from 1 to 10 carbon atoms A group, preferably an alkyl group of 1 to 6 carbon atoms, more preferably 1 to 4 carbon atoms, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, or tert-butyl. X is specified as an integer from 1 to 4.
[0024] In this reaction, a metal atom (M) from a hydride replaces a hydrogen in an alcoholic hydroxyl g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com