Novel 10-cyclohexenyl-phenyl pyrrolobenzodiazepine-3-carboxamides and derivatives thereof; tocolytic oxytocin receptor antagonists
A cycloalkyl and alkyl technology, applied in the field of new tricyclic carboxamides, can solve the problems of lack of oral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] The present invention also provides processes for the preparation of the compounds of the present invention.
[0142] Method of the invention
[0143] Compounds of the invention can be prepared according to one of the general methods outlined below.
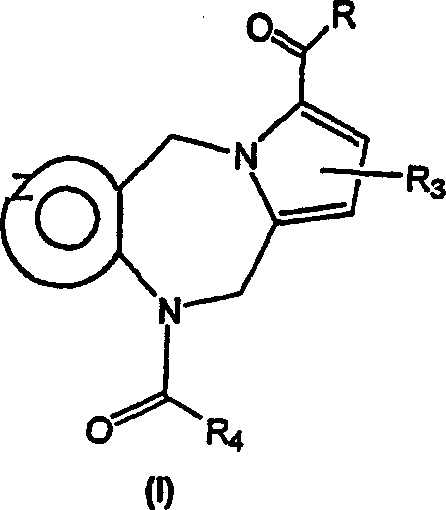
[0144] The compound of general formula (I) can be prepared very conveniently by the method shown in scheme I, wherein R, R 3 and R 4 defined above.
[0145] Option I
[0146]
[0147] According to the preferred method above, in the presence of an organic base such as N,N-diisopropylethylamine (Hunig's base), in an aprotic organic solvent such as dichloromethane at a temperature ranging from -10°C to ambient temperature, The tricyclic diazepines of general formula (1), wherein R 3 and R 4 Reaction with a perhaloalkanoyl halide, preferably trichloroacetyl chloride, as defined above, provides the desired trichloroacetyl intermediate of general formula (2). Subsequent hydrolysis of (2) with an aqueous base such a...
Embodiment 1
[0247]Example 1: 4-chloro-5-cyclohex-1-en-1-yl-2-[(3-{[(2S)-2-(pyrrolidin-1-ylmethyl)pyrrolidin-1- Base]carbonyl}-5H-pyrrolo[2,1-c][1,4]benzodiazepine-10(11H)-yl)carbonyl]phenyl methyl ether
[0248] Step A. Methyl 4-amino-5-chloro-2-methoxybenzoate
[0249] 4-Amino-5-chloro-2-methoxybenzoic acid (50.0 g, 248 mmol) was suspended in methanol (500 mL) and the slurry was cooled to 0°C. Thionyl chloride (54.3 mL, 744 mmol) was then added dropwise over 20 minutes. A clear solution initially formed, which later turned into a white suspension. The reaction was warmed to room temperature and stirred for 3 hours. Methanol was evaporated and the resulting slurry was suspended in ether (1 L). The solid was filtered and rinsed well with ether to give the hydrochloride salt of the title compound (50.9 g). The salt was suspended in 1N sodium hydroxide and stirred vigorously for 30 minutes. Filtration and rinsing thoroughly with water gave the title compound free base as a white solid,...
Embodiment 2
[0293] Example 2: 10-(5-chloro-4-cyclohex-1-en-1-yl-2-methoxybenzoyl)-N-[3-(dimethylamino)propyl]-N -Methyl-10,11-dihydro-5H-pyrrolo[2,1-c][1,4]benzodiazepine-3-carboxamide
[0294] 10-(5-Chloro-4-cyclohex-1-en-1-yl-2-methoxybenzoyl)-10,11-dihydro-5H-pyrrolo[2 , 1-c][1,4]benzodiazepine-3-carboxylic acid (0.200 g, 0.419 mmol), (3-dimethylaminopropyl)methylamine (0.074 ml, 0.503 mmol) , 1-hydroxybenzotriazole (0.062 g, 0.461 mmol) and 1-[3-dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (0.088 g, 0.461 mmol) Mixed with amine-free N,N-dimethylformamide (1.7 mL), followed by the addition of N,N-diisopropylethylamine (110 mL, 0.629 mmol). The reaction was stirred at room temperature for 12 hours, then diluted with ethyl acetate (60 mL), washed with water, saturated aqueous sodium bicarbonate and brine. The combined aqueous washes were saturated with sodium chloride and extracted with ethyl acetate. The combined extracts were dried over magnesium sulfate, filtered and co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com