Controlling method for expression system of T7 induced by laevorotary arabinose
A control method and technology of expression system, which are applied in the control field of L-arabinose-induced T7 expression system, can solve problems such as excessive protein, and achieve the effects of maintaining stability, having economic competitiveness and reducing cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention uses L-arabinose to induce the control method of the T7 expression system, comprising the following steps:
[0028] Construct a strain of recombinant strain, this recombinant strain contains a plastid containing T7 promoter, and the chromosome of this recombinant strain contains T7 gene 1 and araC control gene controlled by araBAD promoter;
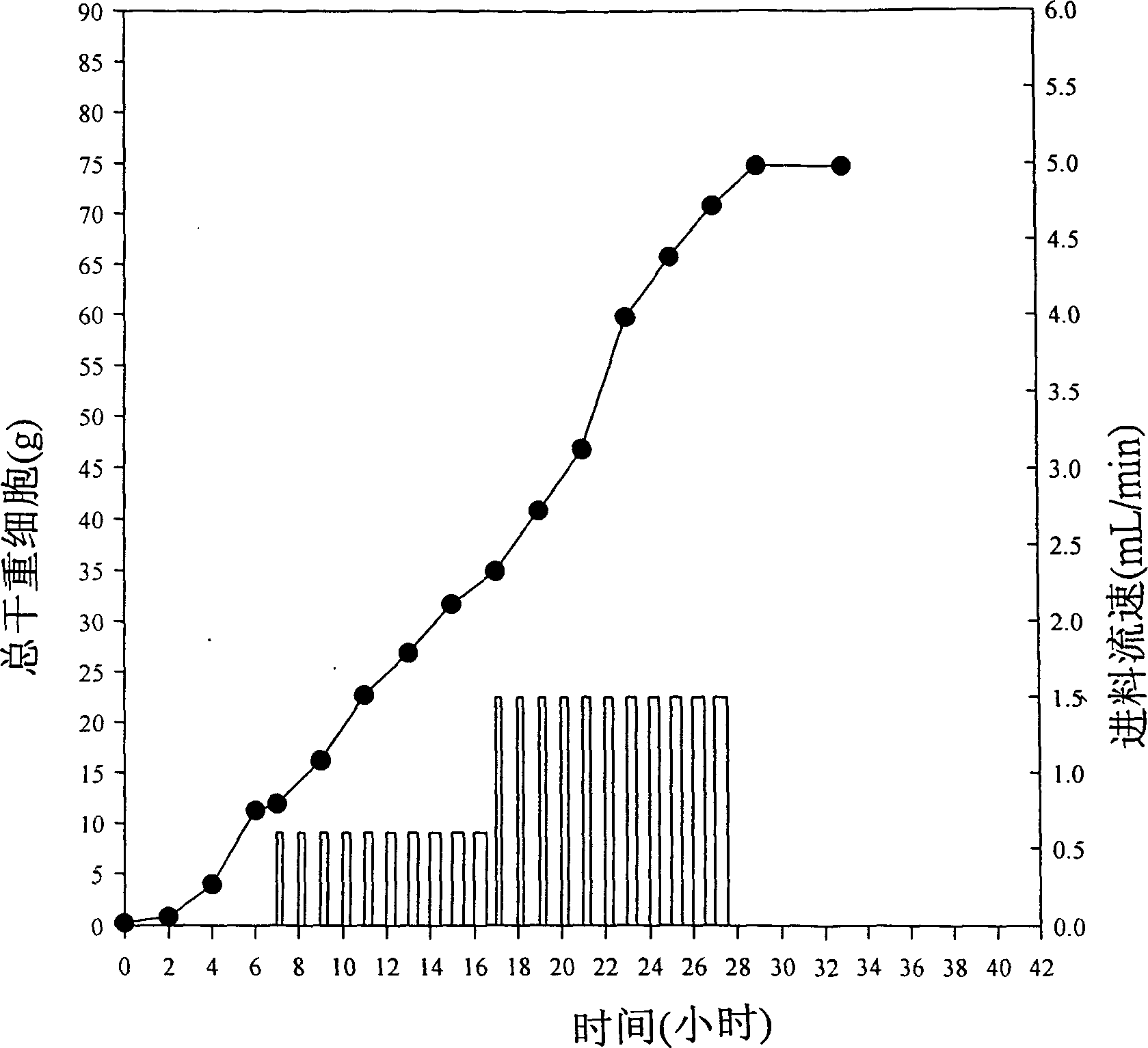
[0029] The recombinant bacterial species is cultivated by a feed batch fermentation method. In the batch fermentation stage, the provided fermentation liquid contains glucose as a carbon source, and in the feed stage, the provided feed liquid contains glycerol and glucose as a carbon source. enabling high cell density fermentation; and
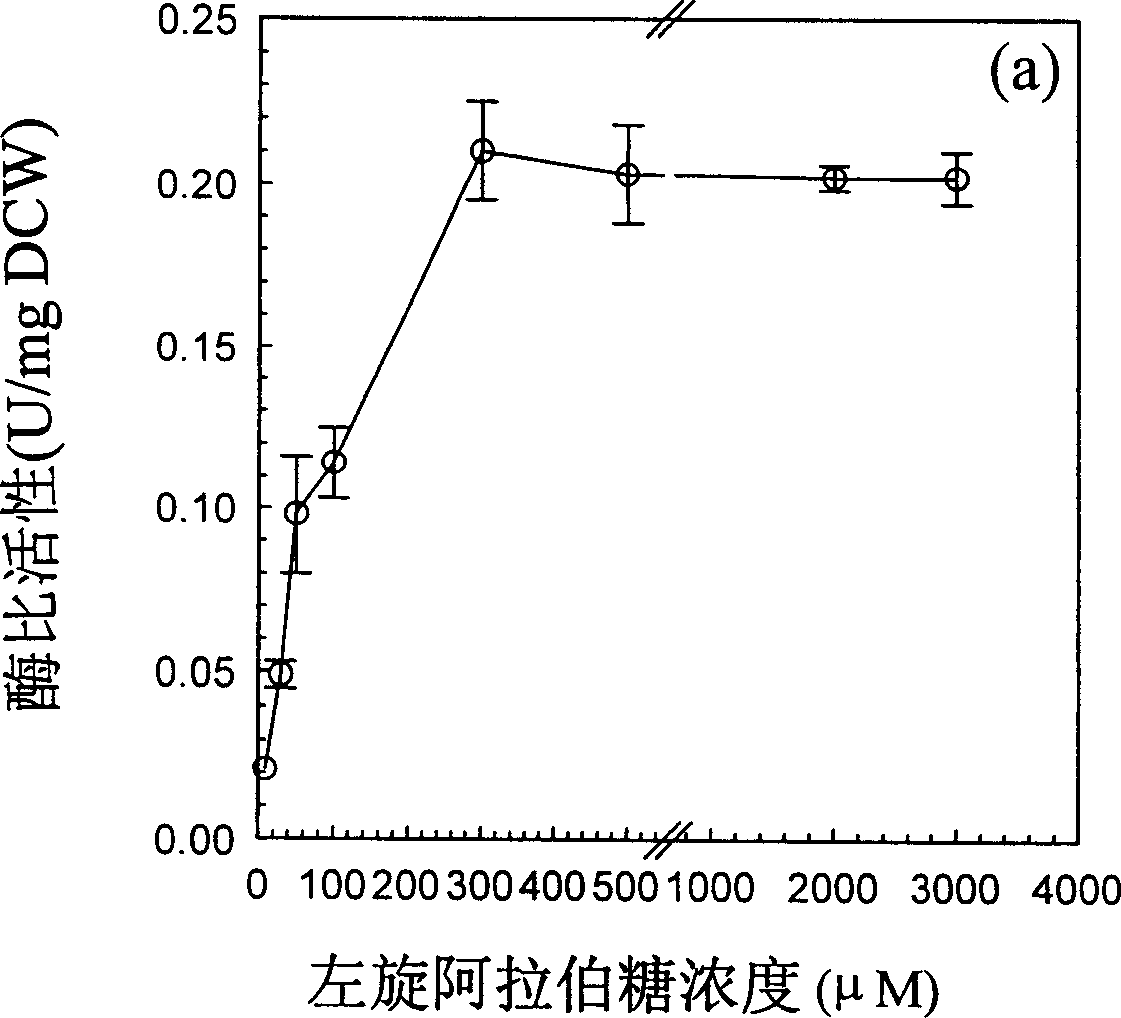
[0030] After the cells reach a certain high density, an inducer containing L-arabinose is added to make the recombinant strain produce a recombinant protein.
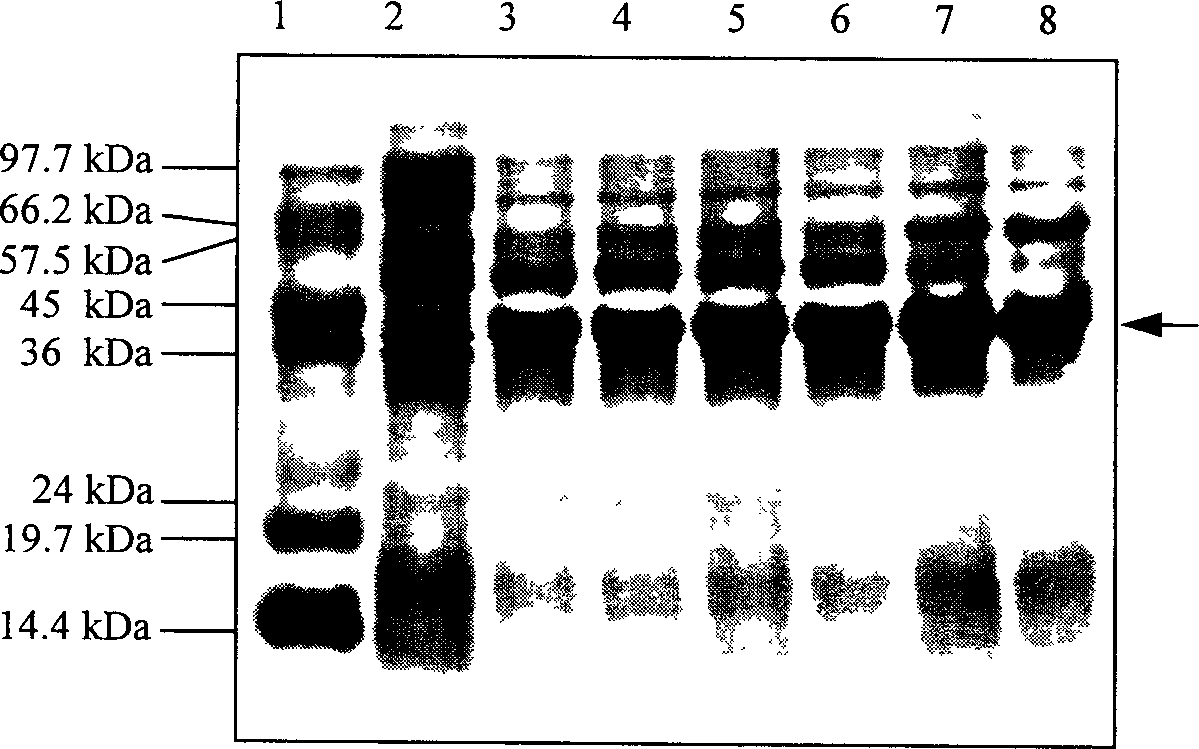
[0031] The control method of the present invention further comprises: the recombinant strain BL21 (BAD) constructed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com