Recombination human Mucl-MBP fusion protein antitumour vaccine and production technology
A fusion protein, anti-tumor technology, applied in the field of recombinant human MUC1-MBP fusion protein anti-tumor vaccine, can solve problems such as weak immunogenicity and toxic T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
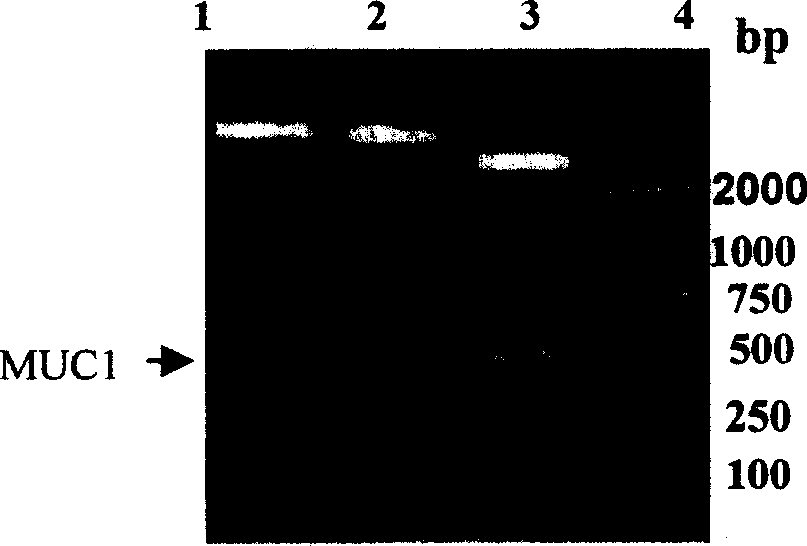
[0097] 1. Construction of MUC1-MBP fusion protein expression vector
[0098] 1. Routine plasmid extraction and purification. (See Molecular Cloning Experiment Guide, J. Sam Brook, edited by Science Press, 1998)
[0099] 2. Separation and recovery of digested fragments.
[0100] (1) Digest pMAL-P2 and PSK-MUC1 plasmids with EcoRI and HindIII for 1-3 hours at 25-37°C.
[0101] (2) The DNA fragments were separated by electrophoresis on 0.7-1.5% agarose gel, and the vector fragment pMAL-p2 and the 450bp target fragment MUC1 were recovered by a freeze-thaw method or a kit.
[0102] (3) Electrophoresis on 0.7-1.5% agarose gel to identify the recovered carrier fragment and target fragment.
[0103] 3. Ligation of pMAL-p2 vector and MUC1 fragment
[0104] pMAL-p2 vector fragment 1-10μl
[0105] MUC1 fragment 5-20μl
[0106] EcoRI 2-4μl
[0107] HindIII 2-4μl
[0108] T4 ligase 1-2μl
[0109] According to the above amount, react overnight at 16°C in a 50-100 μl reaction system....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com