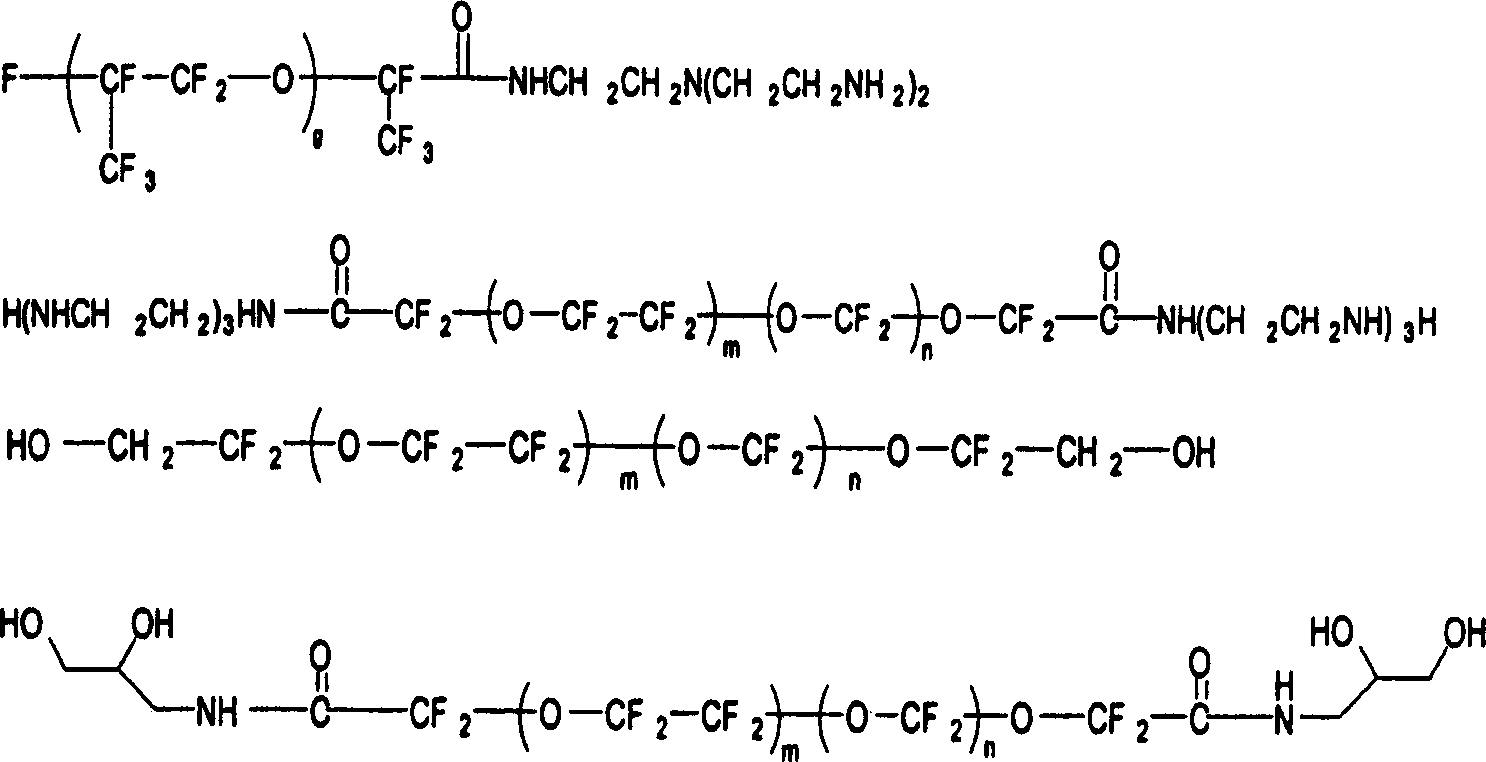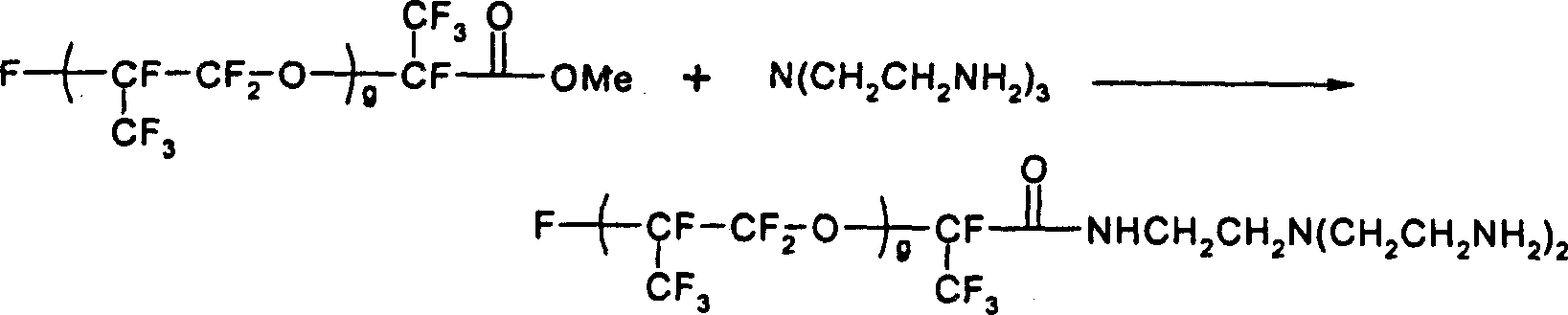High performance capsule for electrophoretic displaying device
An electrophoretic display and capsule technology, applied in the field of novel non-aqueous capsules, can solve the problems of difficulty in controlling the size distribution of capsules, difficult sealing of strong charge control agents, etc., and achieve the effects of improving switching rate and moisture resistance and reducing manufacturing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0096] Preparation of fluorinated isocyanates as shell-forming species in the internal phase
[0097]
[0098] 12 grams of Krytox-alcohol (g = ~6, molecular weight = ~1200, DuPont) was dissolved in 50 grams of 1,1,2-trichlorotrifluoroethane. Within four hours, the resulting solution was added dropwise with stirring to another refluxing solution containing 5.85 g of Desmodur_N3400 (EW=195 from Bayer), 30 g of 1,1,2-trichlorotri Fluoroethane and 5 grams of α, α, α-trifluorotoluene. Reflux was continued for another 10 hours to complete the reaction. After the solvent was removed, a viscous liquid was obtained. Infrared spectrum shows at 1730cm -1 A peak at , which is characteristic of the urethane groups in the product.
[0099] A series of fluorinated polyfunctional isocyanates were synthesized by the same reaction steps from various fluorinated alcohols, including 1H, 1H, 11H-perfluoro-1-undecanol (Aldrich), Zonyl FSO and FSN (Dupont ) and 2,2,3,3,4,4,5,5-octafluoro-1...
preparation example 2
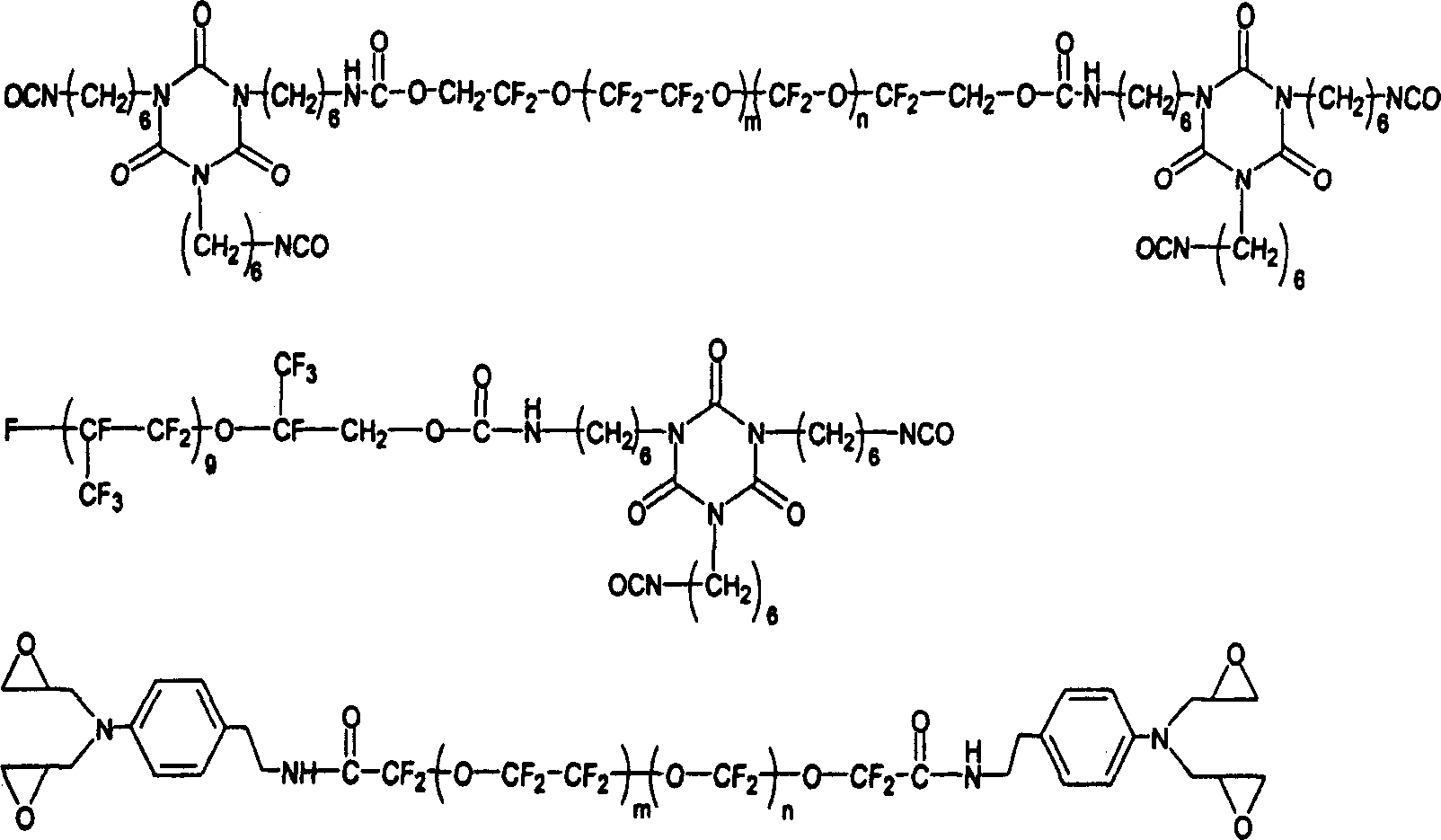
[0102] Preparation of fluorinated epoxides as shell-forming species in the internal phase
[0103] Fluorinated epoxides can be synthesized according to the following reaction pathways. Fluorolink D (from Ausimont) was treated with an excess of sodium hydride and allyl bromide was added to the resulting mixture to produce a fluorinated diene, which was subsequently oxidized with a peracid to form a fluorinated diepoxide.
[0104]
[0105] Alternatively, multifunctional fluorinated epoxides can be synthesized by reacting multifunctional fluorinated alcohols with epichlorohydrin.
preparation example 3
[0107] Synthesis of Fluorinated Amines as Shell Forming Species in the Internal Phase
[0108]
[0109] 17.8 grams of Krytox-methyl ester (molecular weight = about 1780, g = about 10, from DuPont) was dissolved in a solution containing 12 grams of 1,1,2-trichlorotrifluoroethane (Aldrich) and 1.5 grams of α,α, α-trifluorotoluene (Aldrich) in a solvent mixture. At room temperature, under stirring, the solution obtained was added dropwise into another solution after 2 hours. This solution contained 7.3 g of tris(2-aminoethyl)amine (Aldrich). The mixture was then stirred for an additional 8 hours to complete the reaction. The infrared spectrum of the crude product clearly shows that at 1780cm -1 The C=O vibration of the methyl ester disappears, and at 1695cm -1 The C=O vibration of the amide product occurs at . Solvent was removed by rotary evaporation followed by vacuum stripping at 100°C for 4 to 6 hours. The crude product is then dissolved in 50ml of PFS2 solvent (perf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com