Process for preparing serine-rich protein employing cysteine synthase (cysk) gene
A cysteine and serine technology, which is applied in the field of protein preparation, can solve the problems of a lot of time, no results, and difficulty in improving the yield of heterologous proteins.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
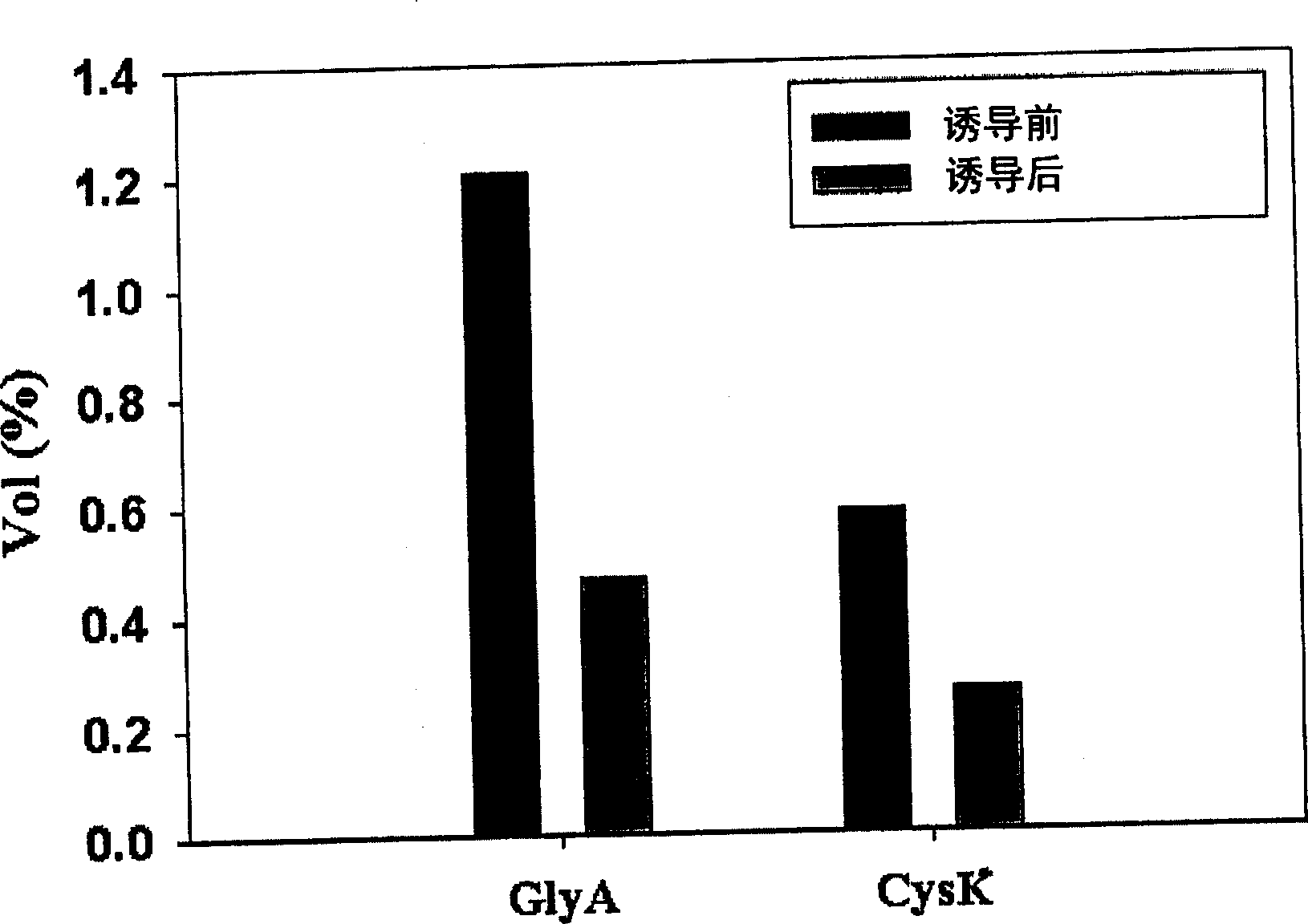
[0033] Example 1: Measuring Physiological Changes of Obesin-Producing Bacteria Using Two-dimensional Electrophoresis
[0034] According to a known method, changes in protein levels before and after overproduction of human obesin in E. coli BL21(DE3)(pEDOb5) were compared using two-dimensional electrophoresis (Hochstrasser et al., Anal. Biochem., 173: 424- 5, 1988; Han et al., J.Bacteriol., 183:301-8, 2001): namely, after E.coliBL21(DE3)(pEDOb5) was pre-cultured, the expression of obesin was induced and cultured at a high concentration . Remove the culture broth before and after induction of expression. Each culture broth was centrifuged at 6,000 rpm for 5 minutes at 4°C, and the precipitate was washed with 500 μl of low-salt buffer (KCl 3mM, KH 2 PO 4 1.5mM, NaCl 68mM, NaH 2 PO 4 9mM) wash. Then, the product was suspended in 200 µl of TE buffer (Tris-HCl 10 mM, EDTA 1 mM). The suspension was sonicated with a sonicator and centrifuged at 12000 rpm for 10 min at 4°C. T...
Embodiment 2
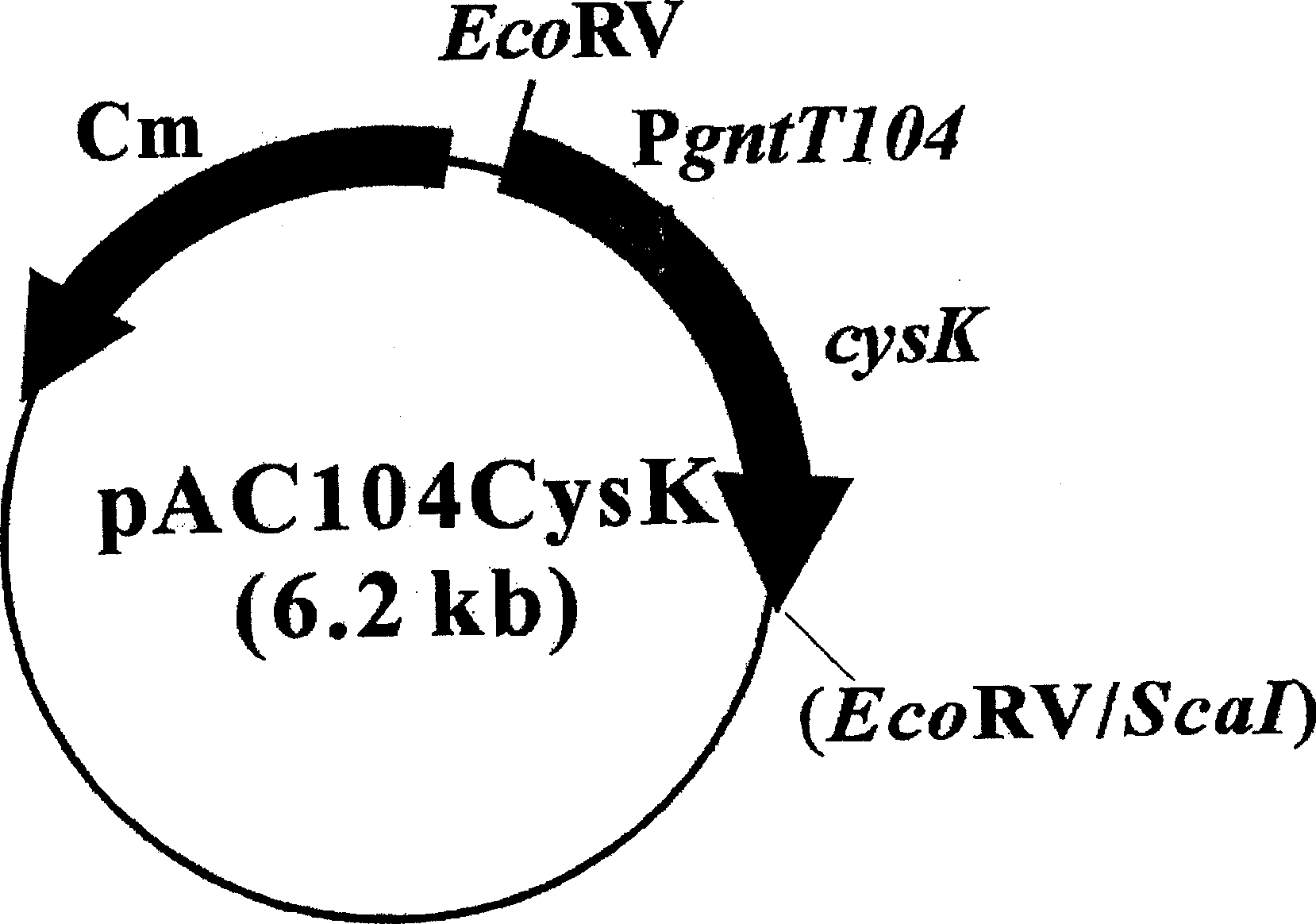
[0038] Embodiment 2: the preparation of the recombinant plasmid that introduces cysK gene
[0039] The preparation of the recombinant plasmid pAC104CysK expressing CysK protein is as follows: First, the E.coliBL21 (DE3) chromosome is used as a template for polymerase chain reaction (PCR), primer 1:
[0040] 5'-gc gaattc atgagtaagatttttgaagataa-3' (SEQ ID NO: 1) and primers
[0041] 2: 5'-gc gaattc tatatactgttgcaattctttctc-3' (SEQ ID NO: 2). At 95°C, perform the first denaturation for 5 minutes; the second denaturation at 95°C for 50 seconds, anneal at 55°C for 1 minute, and extend at 72°C for 1 minute and 30 seconds, repeating 30 cycles; finally at 72°C °C extension for 5 minutes. In this way, the cysK gene digested by the restriction endonuclease EcoRI was obtained, and the obtained fragment was inserted into the plasmid p10499A (Park et al., FEMS Microbiol. Lett., 214:217-22, 2002) having the gntT104 promoter , which was digested with the same restriction enzymes to f...
Embodiment 3
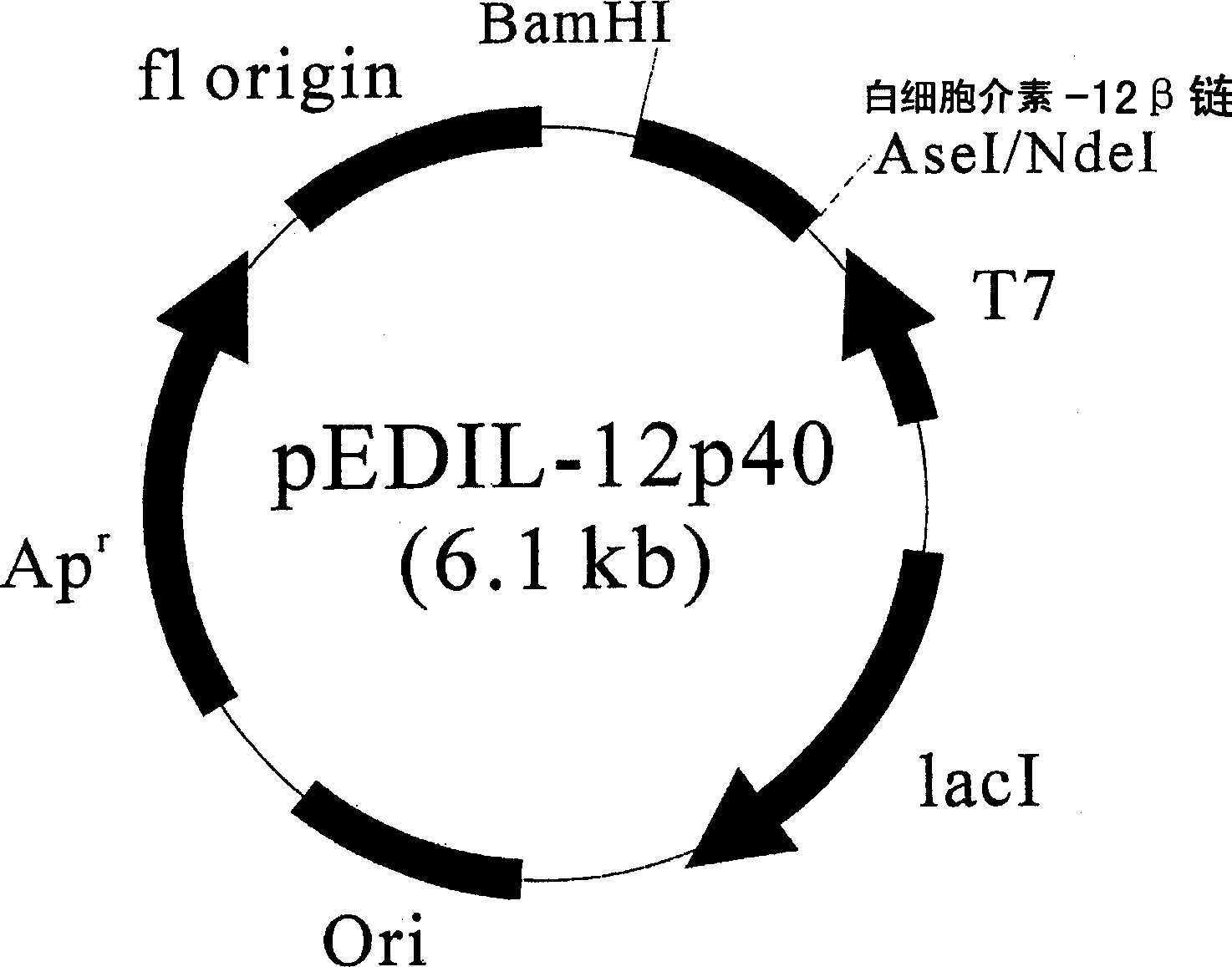
[0042] Example 3: Preparation of recombinant plasmids introducing IL-12p40 gene
[0043] The recombinant plasmid pEDIL-12p40 was prepared as follows to express IL-12p40 (interleukin 12β chain) protein. Use plasmid pUC18 / p40 containing human interleukin β chain gene as template, and primer 3: 5'-ggctagc attaat gatatgggaactgaagaaagat-3' (SEQ ID NO: 3) and primer 4: 5' gcc ggatcc ttattaactgcagggcacaga-3' (SEQ ID NO: 4) was subjected to PCR by the same method as in Example 2 to obtain IL-12p40 gene. The gene was digested with restriction enzymes AdeI and BamHI. The obtained fragment was inserted into the obesin expression vector (Jeong and Lee, Appl. Environ. Microbiol., 65:3027-32, 1999) to constitute pEDIL-12p40, wherein the vector had been digested with restriction enzymes NdeI and BamHI ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com