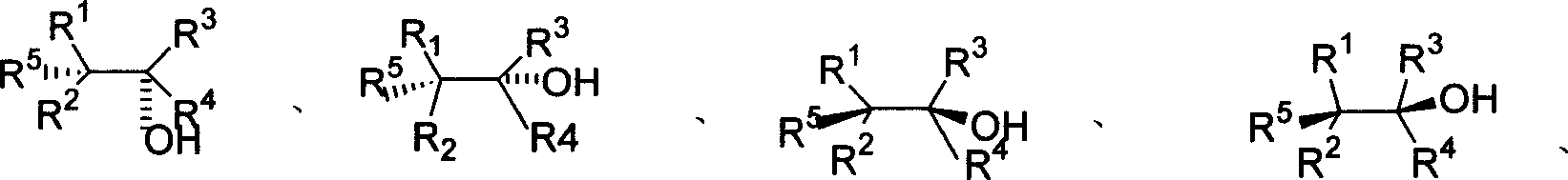
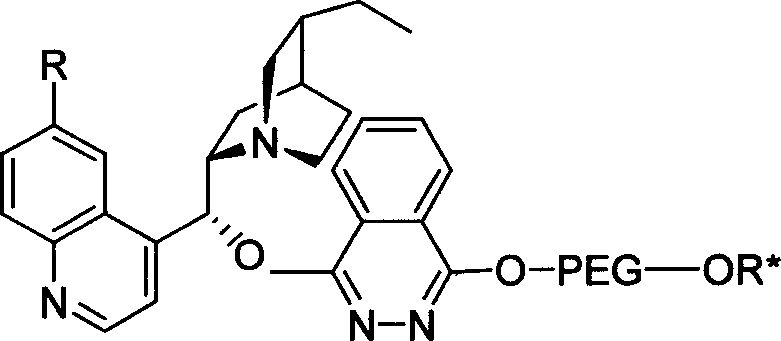
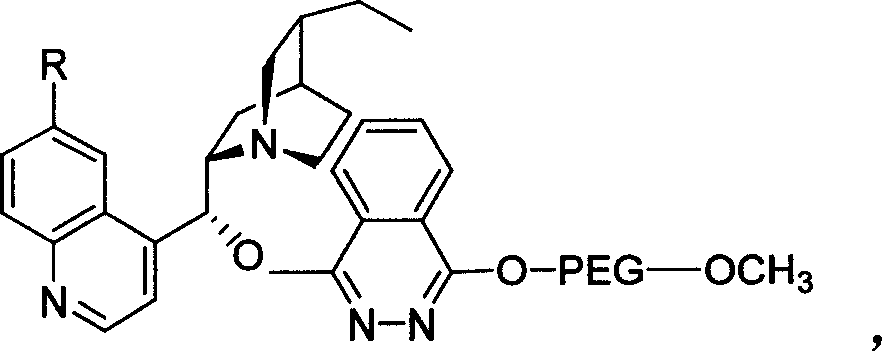
Method for processing asymmetric hydroxylamination and dihydroxylation reaction by use of supported bi-cinchoni alkaloid ligand
An alkaloid, asymmetric technology, used in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve problems such as difficulty in success, difficult recovery, and expensive osmium oxidant.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Ligand connection
[0036] Weigh polyethylene glycol (molecular weight 6000, 12g) and add it to a 100ml dry three-necked flask, under nitrogen protection, add tetrahydrofuran (50ml), then add n-BuLi (3ml) (2.5M in hexane), the addition is complete, Stir at room temperature for 30 minutes, then add Reflux at 78°C until the reaction is complete, then cool, add about 15ml of water, extract with dichloromethane, dry, concentrate, add a very small amount of dichloromethane to dissolve the residue under vigorous stirring, and add diethyl ether under stirring to obtain The white precipitate was stirred for 1 h, then filtered, and the solid was washed three times with a mixture of cold ethanol and ether (1:4), then twice with anhydrous ether, and dried in vacuo to obtain the immobilized ligand. Yield 68%.
Embodiment 2AA
[0037] Embodiment 2AA reaction
[0038] LiOH.H 2 O (1 mmol), ligand (10 or 20 mol%), K 2 OSo 2 (OH) 4 (0.04mmol), added to a solution of tert-butanol / water (1:1) (5ml and 5ml), stirred to obtain a clear solution, cooled to 5°C, added isopropyl cinnamate (1mmol) and CH 3 CONHBr (1 mmol) was stirred at 5°C for 10-15 hours before working up.
Embodiment 3
[0040] The same as example 2 above, but add CH first 3 CONHBr (1mmol), let it stir for 30 minutes, then add isopropyl cinnamate (1mmol), post-treatment with Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com