Production process of freeze dried abalone
A production process and abalone technology, applied in the field of abalone production technology, can solve the problems of hard processing quality, decreased sensory indicators, difficult processing, etc., and achieve the effects of fast freeze-drying process, optimized quality, and guaranteed quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
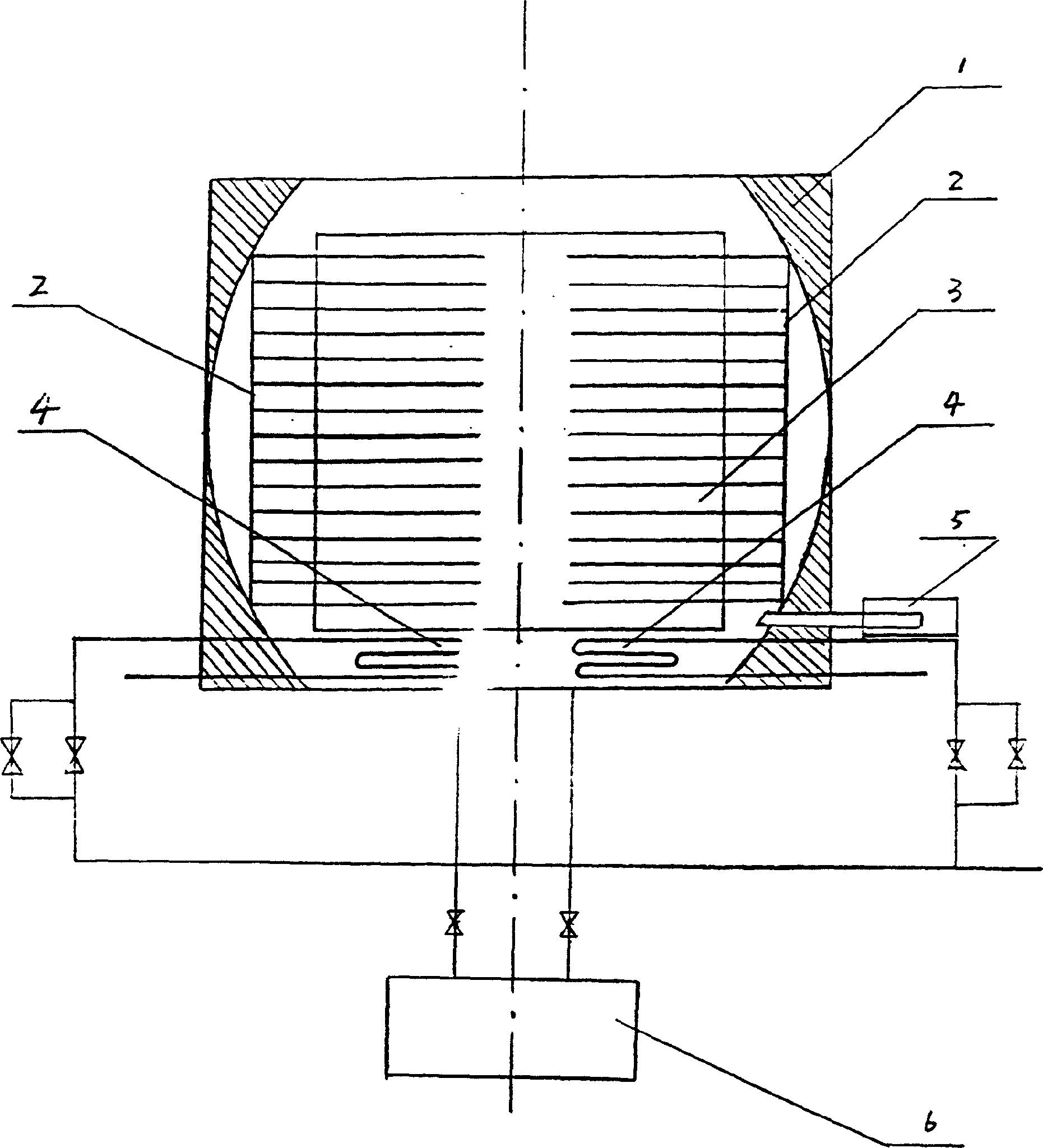
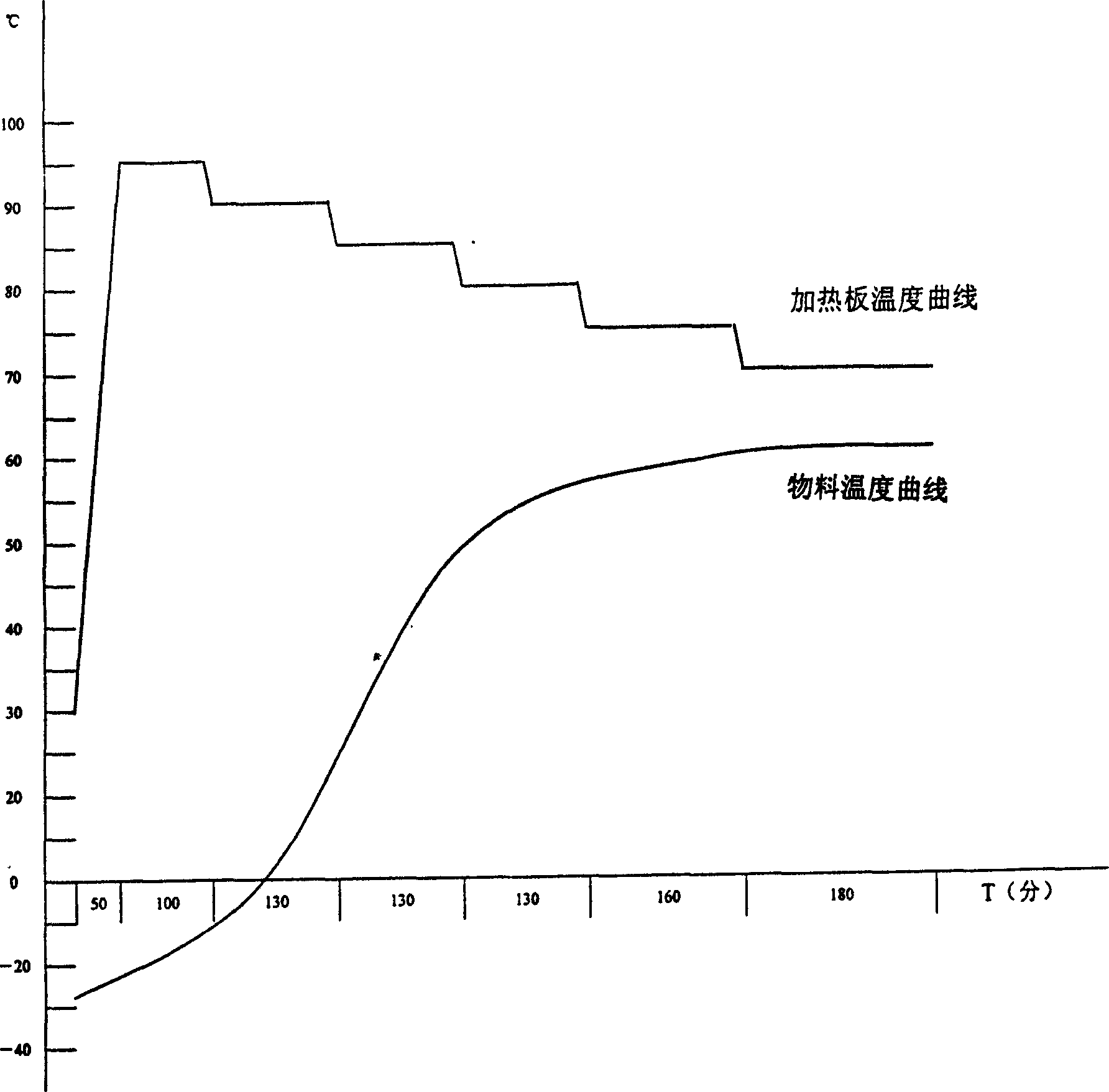
Image
Examples
Embodiment Construction
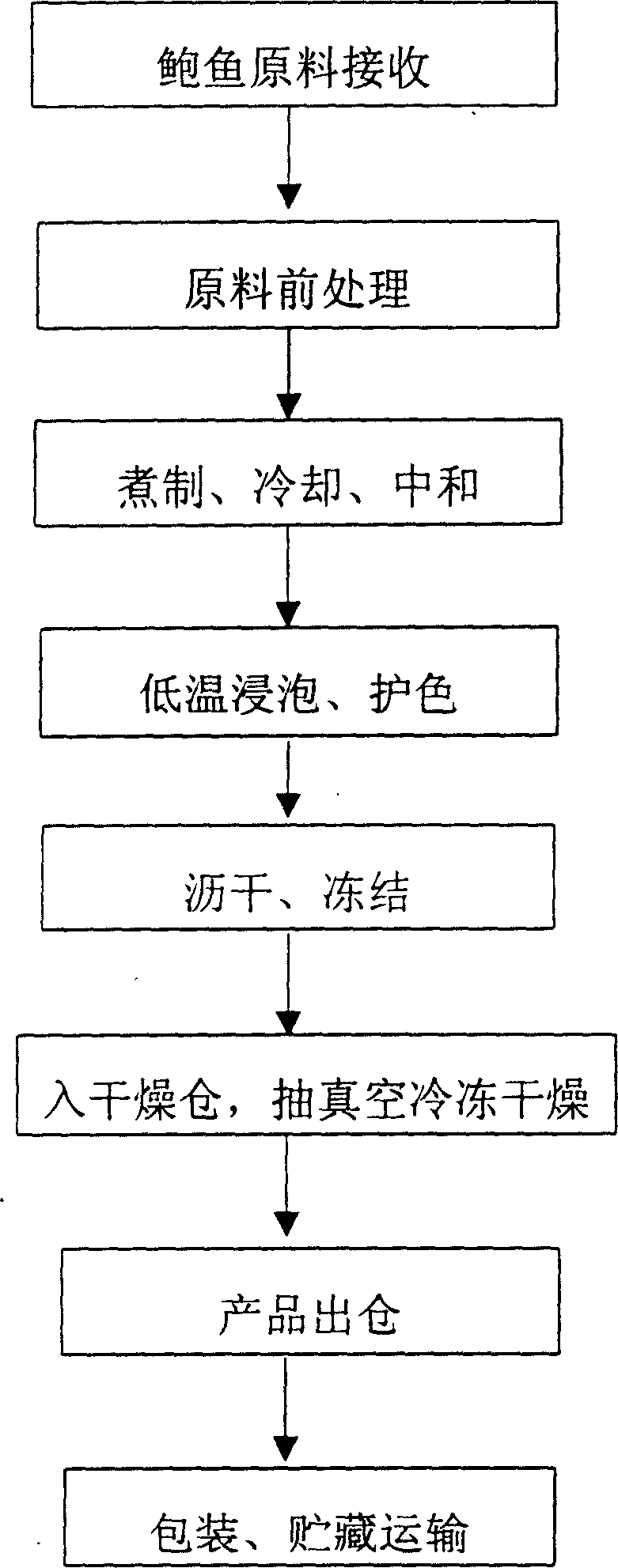
[0010] Technical process of the present invention is described in detail as follows:
[0011] 1. The raw material of abalone is received, and the specification requires about 60g / piece (including shell weight).
[0012] 2. Raw material pretreatment:
[0013] ① Peel off the shell, remove the viscera, remove the mantle, and exfoliate the jaw plate (a component of the abalone mouthparts).
[0014] ②Use 8% refined salt to rub, wash, and scrub until the melanin around the feet is removed. The purpose of using refined salt is also to promote dehydration and convergence of the body as soon as possible, so that the protein can be solidified, which can prevent the shape from cracking caused by the next cooking process.
[0015] ③Cut pattern: Abalone's feet and side shells are rich in muscle mass, and the muscle mass is dense, the type of muscle scars and lines are not obvious, lack of filamentous muscle fibers, and the connective tissue formed between muscle proteins has extremely poo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com