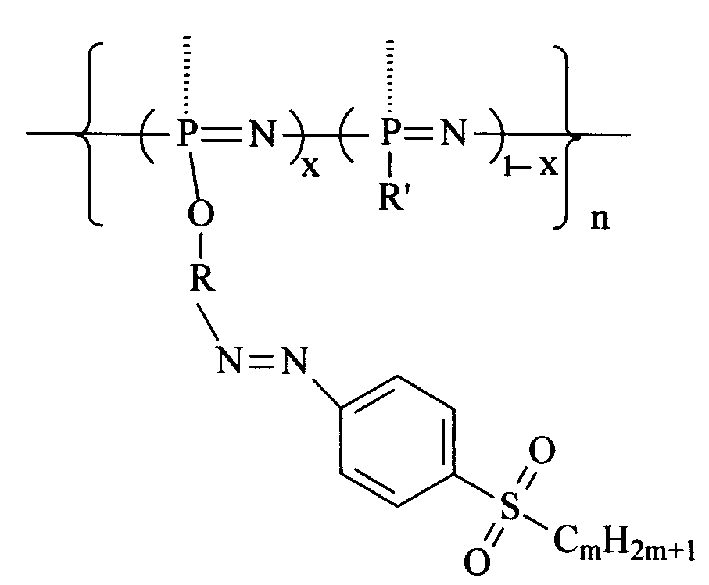
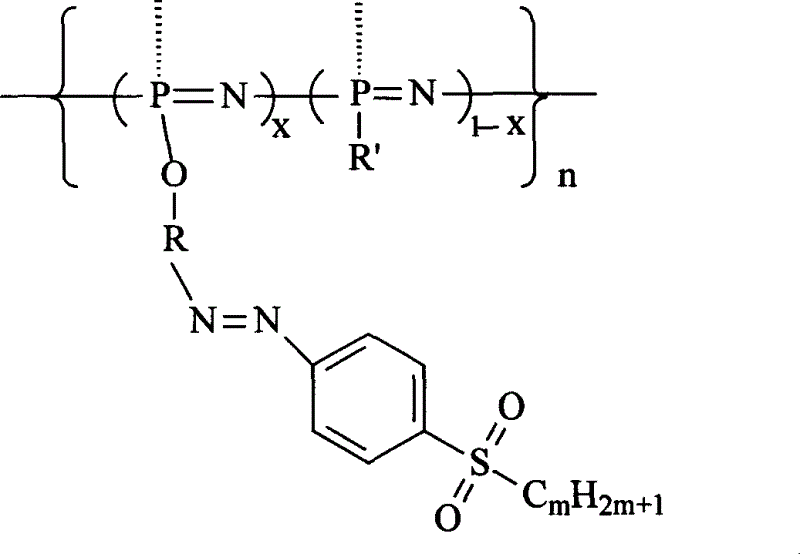
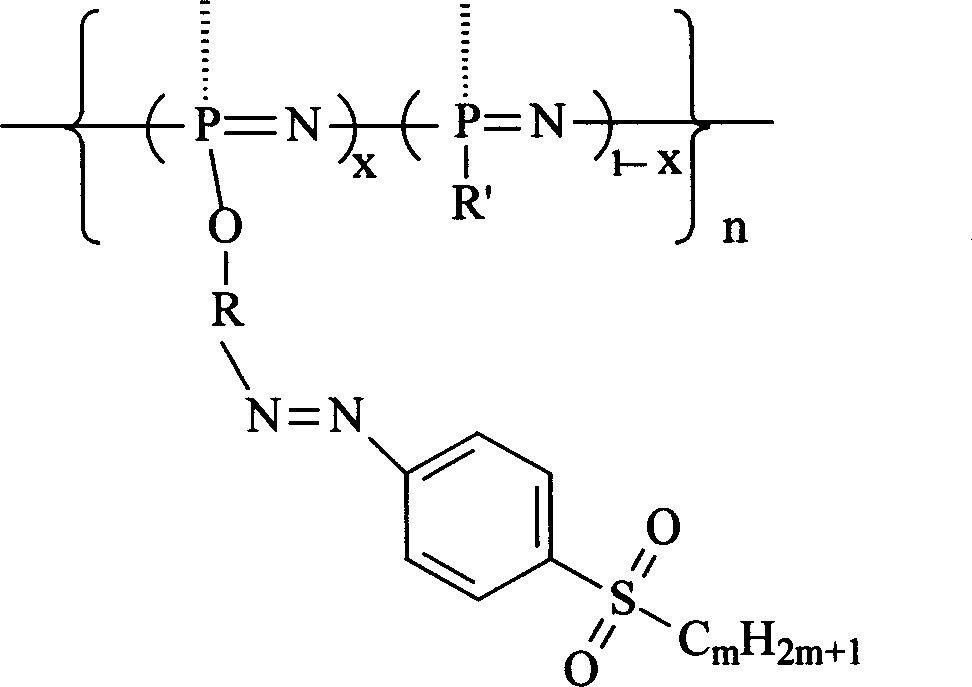
Second-order non-linear optical polyphosphazene connecting sulfuryl-azo chromophor lateral group, and preparing method and use thereof
A second-order nonlinear, sulfone-based azo technology, applied in the direction of organic dyes, etc., can solve the problems of no polyphosphazene, no visible, no indole chromophore, etc., and achieves convenient purification, mild reaction conditions, and simple synthesis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: R is an indole group, R' is an indole group and an ethoxy group, when m is 2, and when x is 0.21, the synthetic route is as follows:
[0016]
[0017] The synthesis method is:
[0018] Dissolve p-ethylsulfonylaniline (2.79g, 0.015mol) in 9.90g of 40% fluoboric acid (about 0.045mol), cool in an ice bath to 0-5°C, and slowly add sodium nitrite (1.04g, 0.015mol) was dissolved in 4mL of water, and the reaction temperature was controlled below 5°C. After reacting for half an hour, quickly filter with suction, and the filter cake was repeatedly washed several times with cold absolute ethanol and absolute ether, and after drying, 4.05 g of light yellow powder was obtained, which was p-ethylsulfonylaniline diazonium fluoroborate, and the yield was 95%, put it in the refrigerator for later use.
[0019] Put N-(2-hydroxyethyl)indole and sodium hydride in a Schlenk tube, add tetrahydrofuran, and then react at 50°C for 10 hours to obtain a tetrahydrofuran solutio...
Embodiment 2
[0022] R is an indole group, R' is a carbazole group, an indole group and an ethoxyl group, m is 2, and when x is 0.07, the synthetic route is as follows:
[0023]
[0024] The synthesis method is: polyphosphazene 3 can be prepared by reacting N-(2-hydroxyethyl)indole, N-(2-hydroxyethyl)carbazole and sodium ethylate with polydichlorophosphazene, and its preparation method and implementation The preparation method of polyphosphazene 1 in Example 1 is generally similar. Weigh 0.16 g of polyphosphazene 3, put it into a test tube, add 1.2 ml of N-methylpyrrolidone, stir at room temperature until the polymer is completely dissolved, and put it in an ice bath. Phenyl phenyldiazonium fluoroborate, reacted under ice bath conditions for 8 hours, then added 0.2 g of anhydrous K 2 CO 3 , keep the ice bath condition, continue to stir for one hour, filter, wash the reaction test tube with tetrahydrofuran, collect the filtrate, remove the tetrahydrofuran under reduced pressure, add 40 ...
Embodiment 3
[0026] R is an indole group, R' is a carbazole group, an indole group and an ethoxyl group, m is 2, and when x is 0.12, the synthetic route is as follows:
[0027]
[0028] The synthesis method is as follows: Weigh 0.16 g of polyphosphazene 3, put it into a test tube, add 1.2 ml of N-methylpyrrolidone, stir at room temperature until the polymer is completely dissolved, and put it in an ice bath. After the temperature of the solution drops to 0°C, add 100 mg of The newly prepared p-nitrophenyl diazonium fluoroborate was reacted for 8 hours under ice-bath conditions, and then 0.23 g of anhydrous K was added 2 CO 3, keep the ice bath condition, continue to stir for one hour, filter, wash the reaction test tube with tetrahydrofuran, collect the filtrate, remove the tetrahydrofuran under reduced pressure, add 40 ml of methanol, an orange-red precipitate can be obtained, filter, collect the orange-red solid, and wash with methanol Several times, vacuum drying at 40°C, the orange...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com