Y-type second-order non-linear optical luminophor contg. triphenylamine, prepn. method and use thereof
A second-order nonlinear, triphenylamine-based technology, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., can solve problems that cannot meet the requirements of human information processing, transmission, and conversion for fast and accurate, and achieve the expansion method and design ideas, the effect of enriching the content of the research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
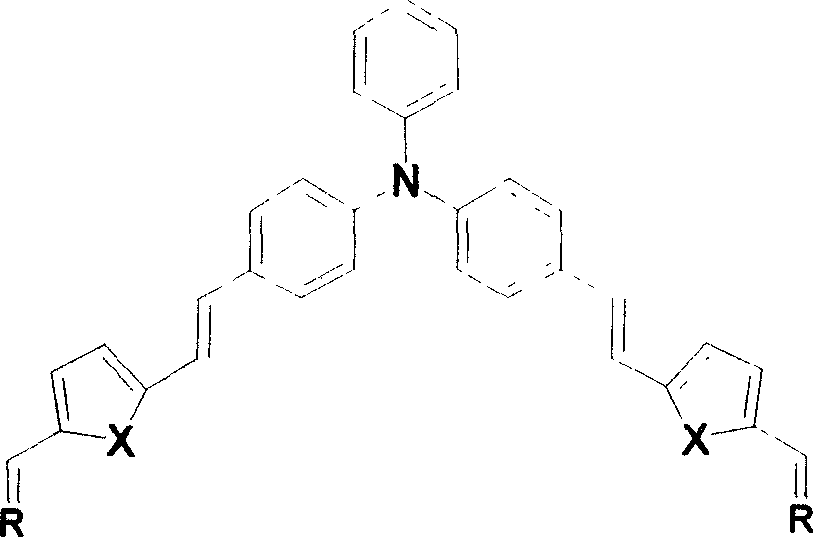
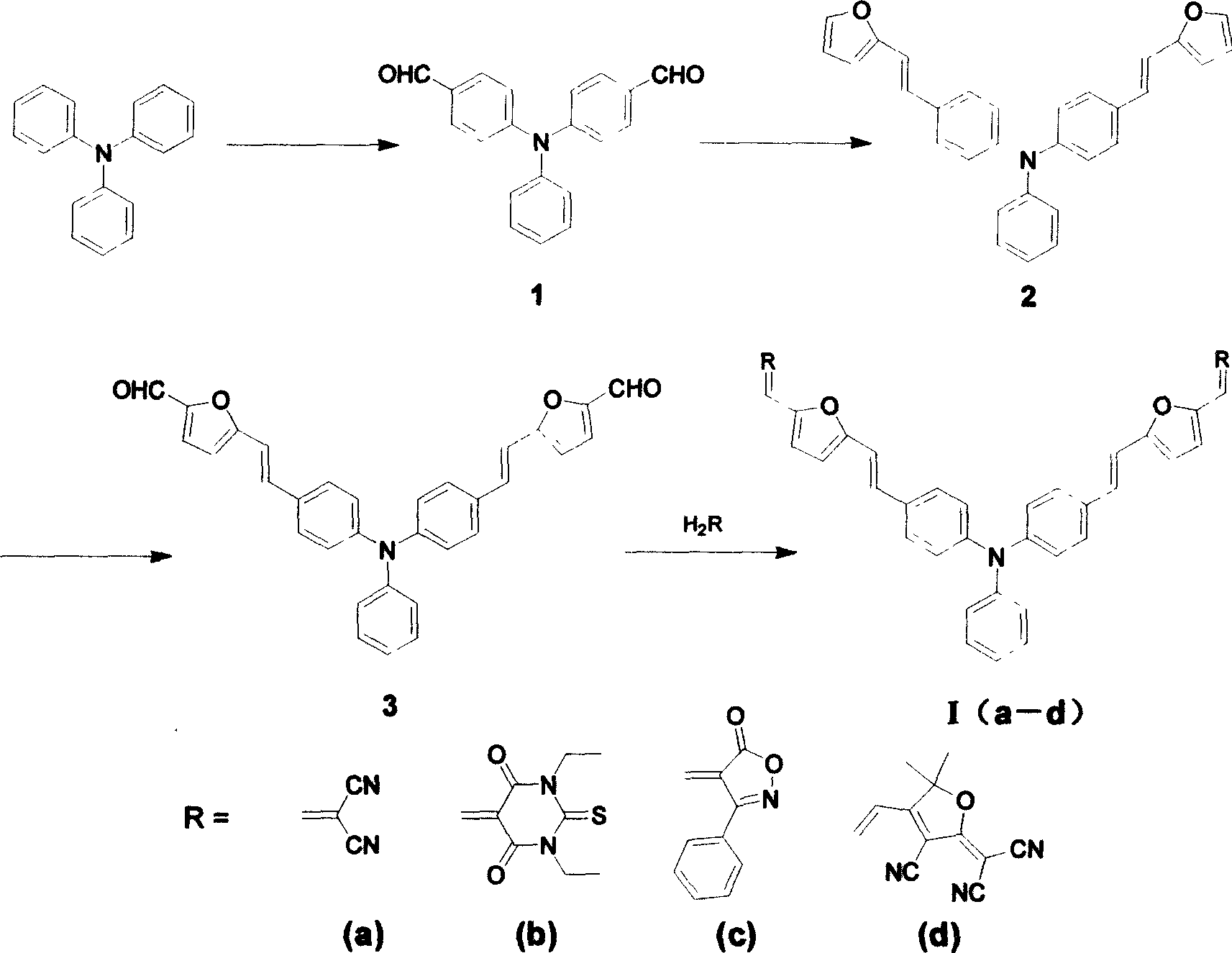
[0015] When X is an oxygen atom, the synthetic route is as follows:
[0016]
[0017] The synthesis method is:
[0018] Synthesis of Triphenylamine Dialdehyde (Compound 1)
[0019] Phosphorus oxychloride POCl 3 Added to DMF, the reaction system was cooled with an ice-water bath and moisture was isolated. After stirring at room temperature for 1 hour, triphenylamine was added. Keep the reaction at 120°C for 12 hours, pour the mixture into ice water (10-15 times the volume of the reaction solution), add 20% NaOH solution to neutralize, a yellow solid is precipitated, and the crude product is purified by silica gel chromatography (eluent : ethyl acetate: petroleum ether = 1: 2), to obtain a bright yellow solid, which is triphenylamine dialdehyde. The molar ratio of triphenylamine, phosphorus oxychloride and N,N-dimethylformamide is 1:3.1-5.9:6.1-9.9. The melting point is 142-143°C. IR: 1692cm -1 (C=O).
[0020] Synthesis of compound 2
[0021] Weigh bromofurylmethyltr...
Embodiment 2
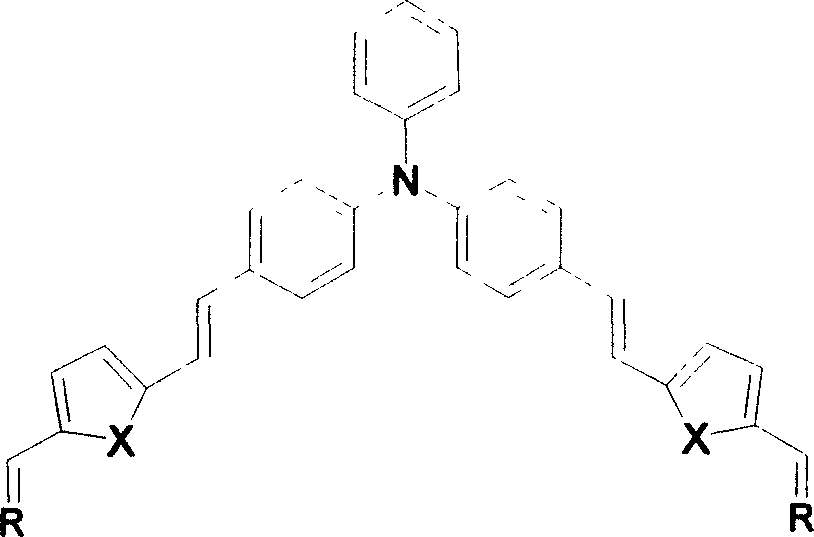
[0033] When X is a sulfur atom, the synthetic route is as follows:
[0034]
[0035] The synthesis method is:
[0036] Preparation of Bromothiophenemethyltriphenylphosphine
[0037] Weigh 2-methylthiophene (5.01g, 50mmol), add in 250ml round bottom flask, then add carbon tetrachloride 150ml, NBS powder (10.7g, 60mmol), BPO powder 0.1g, heat to CCl 4 After reflux for 2h, the reaction solution gradually changed from a light yellow solution to a darker yellow. The reaction solution was filtered and washed with CCl 4 Wash the filter residue and collect the resulting CCl 4 solution, spin off CCl 4 A yellow oily liquid was obtained, to which water was added to remove water-soluble impurities, and CHCl 3 Extraction, adding anhydrous Na 2 SO 4 Let dry overnight. Filter out Na 2 SO 4 , transfer the resulting yellow solution into a 250ml flask, add PPh 3 (10.7g, 50mmol) and CHCl 3 100ml, heating and stirring, to CHCl 3 Reflux, stop heating after 2h of reaction. During t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com