Mannuronic acid and guluronic acid imbedded oligosaccharin and preparation method therefor
A technology of guluronic acid and mannuronic acid, applied in oligosaccharides and other directions, can solve problems such as weak glycosidic bonds, difficult to obtain, and influence research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
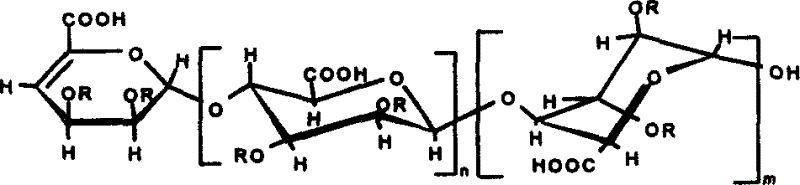
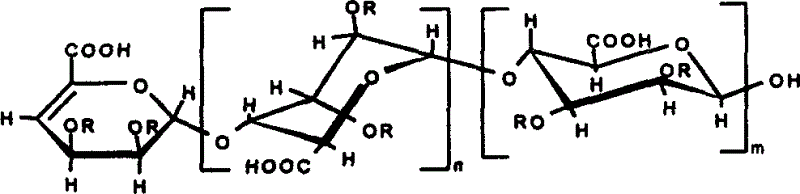
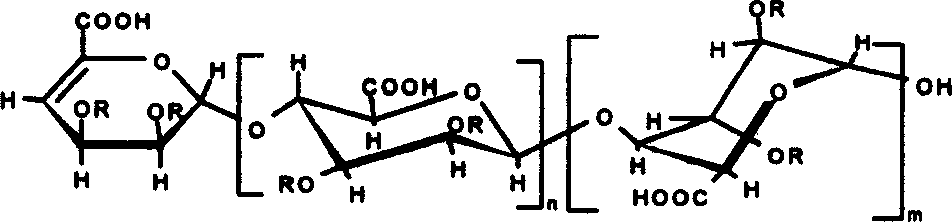
[0011] Prepare alginate into a 5% (weight percent concentration, the same below) aqueous solution, add alginate lyase (Vibro Sp.510) to react, heat in a boiling water bath to inactivate the enzyme, remove precipitated impurities after centrifugation, and The obtained supernatant was separated and purified by gel permeation chromatography, the packing material was Bio-Gel (Bio-Gel) P6 and the strong anion exchange chromatography packing material was Spherisorb SAX, and the non-reducing end had a double bond of mannuronic acid and ancient glucuronic acid mosaic fragment oligosaccharide; and introduce SO at the 2 and 3 positions of the sugar ring 3 Na or CH 3 or PO 3 Na 2 . Its structural formula is:
[0012]
[0013] or
[0014]
[0015] In the formula, n=1-19, m=1-19, n+m3 Na or CH 3 or PO 3 Na 2
[0016] The concentration of the alginate aqueous solution in the present invention can be 1-10%. The alginate lyase used is a specific alginate lyase. In addition to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com